Much of the research on the coronavirus disease 2019 (COVID-19) therapeutics has been focused on essential structures such as the virus-encoded spike protein, main proteases, as well as ribonucleic acid (RNA) polymerase proteins. Obtaining high-resolution analyses of these structures provides a mechanical insight into their function and assists in using these structures as targets in the development of both vaccines and anti-viral therapeutics.
A better understanding of these structures will also allow scientists to effectively overcome the challenges presented by emerging variants and the consequent rise in infection rates that follow. To this end, a recent study published in Nature Structural & Molecular Biology discusses the structures of the SARS-CoV-2 ORF3a using cryo-electron microscopy (cryo-EM).
-2.jpg) Study: Cryo-EM structure of SARS-CoV-2 ORF3a in lipid nanodiscs. Image Credit: Design_Cells / Shutterstock.com
Study: Cryo-EM structure of SARS-CoV-2 ORF3a in lipid nanodiscs. Image Credit: Design_Cells / Shutterstock.com
ORF3a
The virally encoded open reading frame 3a (ORF3a or 3a) is highly conserved within the Betacoronavirus subgenus Sarbecovirus, which includes the severe acute respiratory syndrome (SARS) coronavirus, which is the virus that was responsible for the 2002 outbreak, and other bat-related coronaviruses. By investigating the role of 3a in these and future coronaviruses, scientists can better understand how to target and control these viruses when they emerge in future outbreaks.
Previous studies have observed the effect of SARS and the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in patients using biopsies and other tests. In these studies, 3a expression has been identified in infected tissue of SARS patients, whereas anti-3a antibodies have been found in the plasma of SARS-CoV-2 patients.
Importantly, SARS 3a is associated with inflammasome activation as well as apoptotic and necrotic cell death. Comparatively, the SARS-CoV-2 3a has been implicated in apoptosis and the inhibition of autophagy in vitro. The genomic deletion of 3a in mouse models of both SARS and SARS-CoV-2 has been found to reduce both virus volume and morbidity in mice.
3a is known as one of three viroporins that are encoded by the SARS-CoV-2 genome, along with the envelope protein E and ORF8a. Viroporins are small and mostly hydrophobic multifunctional viral proteins that can alter cellular membranes. This alteration of host cells can lead to the release of viruses from infected cells, further enhancing the virus's spread.
3a has been suggested to form an ion channel, with the 3a of SARS being reported to form an emodin- and barium (Ba2+)- sensitive cation channels. Despite these findings, the specific role of 3a within the pathogenesis of diseases remains unknown, which may be due to the lack of insight into how 3a works.
To tackle this issue, the researchers of the current paper have utilized cryo-electron microscopy (cryo-EM) to determine the structures of SARS-CoV-2 3a. This paper aimed to assess the activity of 3a as an ion channel in vitro using electrophysiology and fluorescent ion flux assays.
Study findings
In the current study, the researchers determined the structures of dimeric and tetrameric SARS-CoV-2 3a in lipid nanodiscs. The nanodiscs were made from the scaffold protein MSP1E3D1 and a mixture of DOPE, POPC, and POPS lipids. The structures of these nanodiscs were subsequently analyzed by cryo-EM at 2.9- and 6.5-Å resolution.
SARS-CoV-2 3a was found to adopt a novel dimeric fold, forming a non-selective calcium (Ca2+) permeable cation channel. When viewed from the membrane plane, 3a can be perceived to be approximately 70 Å tall with a 40-Å high transmembrane region and a cytosolic domain which extends 30 Å out of the membrane.
The transmembrane region of 3a consists of three helices per protomer with the N terminus positioned on the extracellular side and the C terminus on the cytosolic side. The transmembrane helices (TMs) can also be seen to trace the circumference of an ellipse from the extracellular side. The TMs are structured against each other and are joined by short intracellular and extracellular linkers.
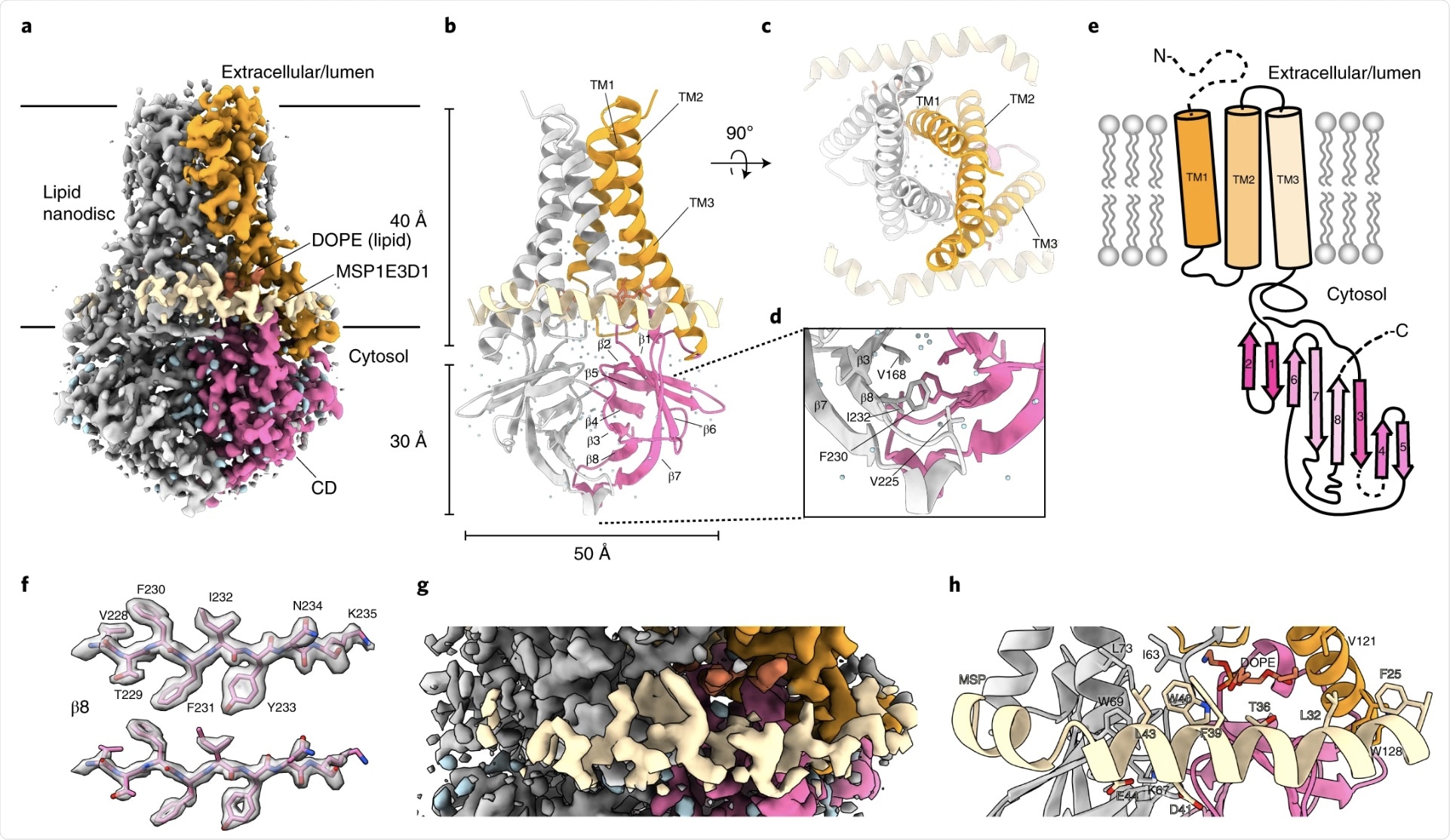 a, Cryo-EM map of the 3a dimer in MSP1E3D1 nanodiscs at 2.1-Å nominal resolution, viewed from the membrane plane. One subunit is colored gray, and the second subunit is colored with transmembrane region in orange and the CD in pink. Density from the nanodisc MSP1E3D1 is colored tan, and the DOPE lipid is coral. b,c, Model of dimeric 3a viewed from the membrane (b) (as in a) and from the extracellular or lumenal side (c). d, Enlarged view of the interaction between subunits in the CD with residues forming the hydrophobic core indicated. e, Schematic of a 3a monomer. Secondary structure elements are indicated, and unmodeled termini and a five-amino-acid β3–β4 loop are shown with dashed lines. f, Cryo-EM density and model for a selected strand from the CD at two different thresholds. g, Enlarged view of density in the MSP1E3D1-interaction region. h, Model in the same region as g with key residues in the area displayed as sticks.
a, Cryo-EM map of the 3a dimer in MSP1E3D1 nanodiscs at 2.1-Å nominal resolution, viewed from the membrane plane. One subunit is colored gray, and the second subunit is colored with transmembrane region in orange and the CD in pink. Density from the nanodisc MSP1E3D1 is colored tan, and the DOPE lipid is coral. b,c, Model of dimeric 3a viewed from the membrane (b) (as in a) and from the extracellular or lumenal side (c). d, Enlarged view of the interaction between subunits in the CD with residues forming the hydrophobic core indicated. e, Schematic of a 3a monomer. Secondary structure elements are indicated, and unmodeled termini and a five-amino-acid β3–β4 loop are shown with dashed lines. f, Cryo-EM density and model for a selected strand from the CD at two different thresholds. g, Enlarged view of density in the MSP1E3D1-interaction region. h, Model in the same region as g with key residues in the area displayed as sticks.
In SARS-CoV-2 3a, both reducing agents and a C133A mutation were found to cause a loss of oligomerization, membrane localization, and ion channel activity. However, with the low expression of the C133A mutation, these results may have been due to protein destabilization.
When investigating whether non-selective cation inhibition blockers inhibit 3a activity in liposomes, the researchers observed that ruthenium red, a 786-Da polycationic dye, blocks 3a activity in current recordings and Ca2+ influx. However, other tests on low-affinity blockers of SARS 3a channel activity, such as Ba2+ and emodin, did not inhibit SARS-CoV-2 3a activity. This finding was confirmed through a cryo-EM observation, where the researchers did not note the occurrence of emodin binding.
Conclusion
These findings provide the foundation for further research into the mechanisms of 3a channel gating and conduction. The study mentions that if 3a exerts channel activity within cells, this viroporin could play a significant role in promoting viral maturation through the inhibition of autophagy and disruption of lysosomes. Since the 3a of SARS-CoV-2 has been shown to stimulate programmed cell death within cells in culture, combined with the observation that calcium influx through 3a might be an activation trigger for calcium-dependent caspases and apoptosis, the role of calcium permeability in 3a could be a significant therapeutic target.
The researchers also described the possibility that the expression of a calcium-permeable channel could affect lung homeostasis and, in turn, the pathogenesis of SARS-CoV-2 infection. This provides further evidence supporting the use of 3a as a target for the treatment of SARS-CoV-2, as well as other bat-related coronaviruses, due to the role of 3a in the viral life cycle.