How do viruses reorganize host cells with spatial and molecular specificity? Researchers from the Netherlands provided new insights in a recent study posted to the bioRxiv* preprint server, where they use optical nanoscopy to look at severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-triggered changes in primary human airway cells.
This study provides a better understanding of viral pathogenesis and how the process repurposes the subcellular architecture of the host cell. In this work, the researchers overcome limitations in techniques such as Electron Microscopy (EM) and immuno-electron microscopy (immuno-EM).
While immuno-EM provides a localized view of proteins at the ultrastructural level, it is impaired by low labeling efficiency, a small field of view, and a lack of volumetric information. Likewise, combining the large field of view and specific labeling offered by optical immunofluorescent microscopy with EM-based ultrastructural analyses is labor-intensive and limited by the resolution of conventional optical microscopy.
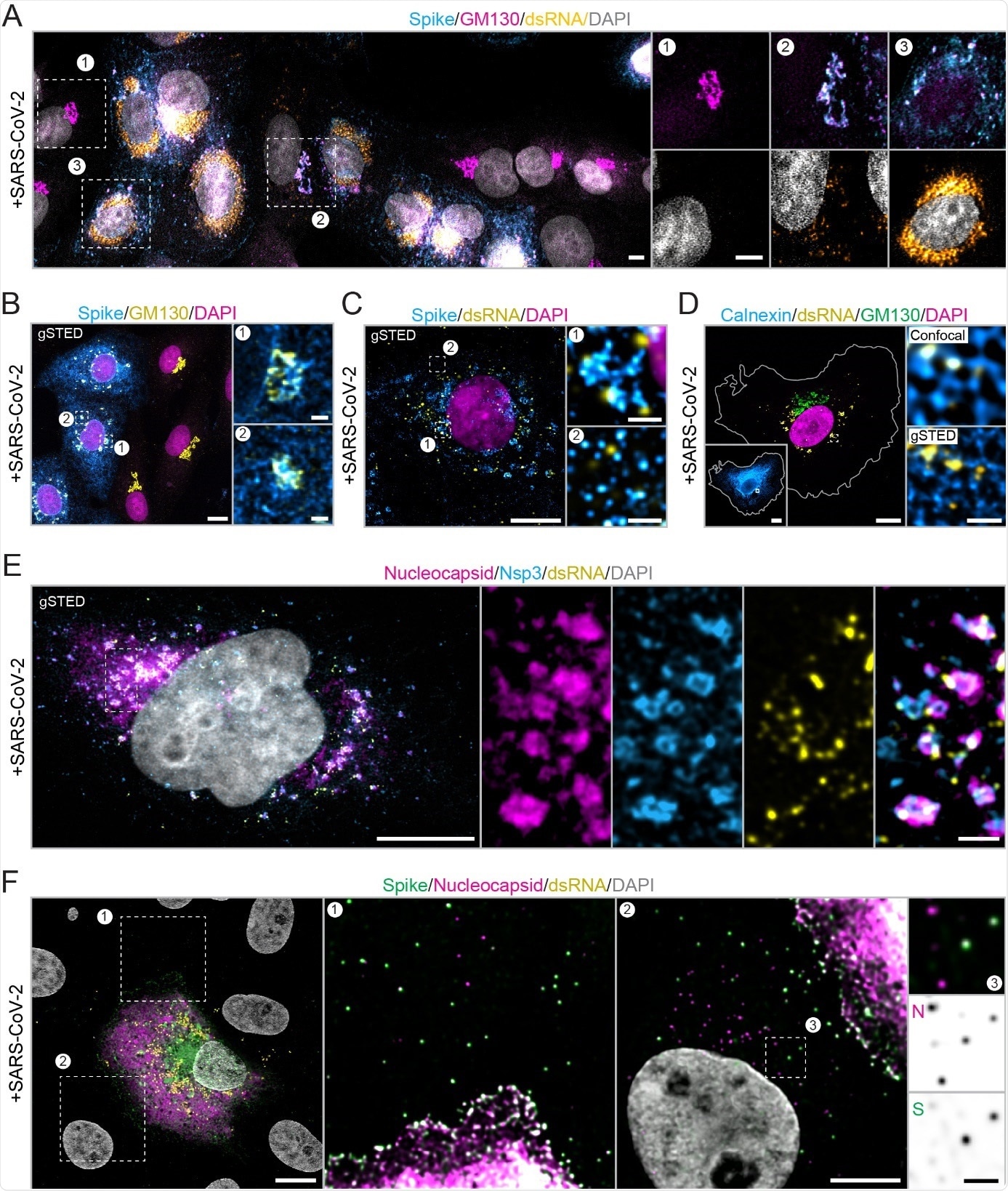
Here, the researchers used Stimulated Emission Depletion (STED) microscopy to study the spatial organization of viral structural proteins, nonstructural proteins, and dsRNA (double-stranded RNA) at <100 nm resolution. Additionally, they employed a novel type of Expansion Microscopy (Tenfold Robust Expansion (TREx) Microscopy) combined with both selective and non-selective protein stains to visualize the morphological changes of airway epithelium induced by SARS-CoV-2 infection.
“We use diffraction-unlimited fluorescence microscopy to analyze how SARS-CoV-2 infection exploits and repurposes the subcellular architecture of primary human airway cells.”
Using these techniques enabled the researchers to see the volumetric ultrastructure of the epithelium with the specific labeling of viral proteins. The researchers also visualized the extensive intracellular reorganization, including Golgi fragmentation, the emergence of large multivesicular bodies containing spike-positive vesicles (MVBs), clustering of cilia, and apical membrane remodeling.
“As such, this work reveals the power of volumetric fluorescence nanoscopy for studying SARS-CoV-2-induced cytopathic effects in primary cell culture models,” said the researchers in the paper.”
In a physiologically relevant model system, the researchers characterized the SARS-CoV-2 infection and subcellular localization of viral entry factors in a human airway model. The primary human airway cells model is used here because the upper respiratory system is the primary site of droplet-based infection by airborne viruses. This study revealed the 3D ultrastructure of human airway cells.
Nanoscopic visualization of the SARS-CoV-2 infection cycle in Vero E6 cells
The researchers collected the adult human airway cells from nasal brushings of healthy volunteers without respiratory tract symptoms and cultured the Human nasal epithelial cells (HNEC) used in the study.
The researchers used multicolor immunofluorescent STED microscopy to visualize the viral uncoating during entry into the host cell, the subsequent subdomain architecture changes of the replication organelles, and the distribution of host entry factors on cilia. They used general protein and membrane labels to resolve cellular ultrastructures in the complex 3D samples.
“Our TREx protocol yields a single-step isotropic tenfold resolution improvement, which enables volumetric multicolor super-resolution imaging of thick human-derived samples on a conventional confocal microscope. This facilitates volumetric optical imaging of the 3D ultrastructure of human airway cells, in combination with markers for specific proteins.”
In addition to rich microscopic and nanoscopic images of the SARS-CoV-2 infected cells and their characterization, six videos of TREx imaging of human airway cells infected with SARS-CoV-2 stained for different proteins supplement the paper.
Notably, the researchers identified large organelles emerging upon infection as atypical CD63 (an intraluminal vesicle marker)-positive MVBs. Previously reported as lysosomes enriched with the virus, this study identified these as MVBs containing a subset of intraluminal virion particles (spike-positive) in the SARS-CoV-2-infected cells which might represent the viral particles en route to either lysosomes or the plasma membrane.
“Our work demonstrates the importance of combining (three-dimensional) ultrastructural imaging with selective labeling.” observed the researchers, highlighting the importance of the tool used in the study.
“Ultrastructural analyses of viral infection are hampered by the disruption of recognizable morphological features in late stages of viral infection, which precludes identification based on morphology and necessitates immunolabeling of specific proteins to obtain a molecular understanding.”
The viral egress is a crucial stage during pathogenesis and a strategic target in treatment options to block the MVBs. Here, the researchers observed the fusion of MVBs containing virus-like intraluminal particles with the plasma membrane. They explained that these MVBs (containing complete virions and overexpressed spike) could be an intermediate for viral trafficking between the Golgi/ERGIC (ER-Golgi intermediate compartment) and lysosomes, allowing extensive intracellular reorganization.
In addition to the intracellular reorganizations in the host cell upon infection with SARS-CoV-2, the researchers observed the following morphological rearrangements at the cell surface:
- overall surface roughening
- emerging spike-positive filopodia
- severe apical remodeling
- spike accumulation on actin-rich microvilli
- clustering of ciliary tips in the infected cells as well as the neighboring uninfected cells.
The researchers discussed these observed changes and the associated interactions.
Interestingly, it is known that viral tropism, the degree of infection, and the replication efficiency depend on the airway model used (nasal, tracheal, or bronchial) and the differences in culture protocols, donor to donor variation, and the specific SARS-CoV-2 strain used for infection.
Under such possible experimental variations, the researchers pointed out that the culture protocol used in the study allows for the robust growth and differentiation of basal cells that were obtained from nose brushes of healthy donors and patients suffering from COVID-19. And the nanoscopy approaches developed here could aid the comparison of HNECs derived from asymptomatic and severely affected COVID-19 patients.
The imaging tools, protocols, and new insights into the SARS-CoV-2 infection and COVID-19 progression may help develop therapeutics and study human-derived tissue models.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
- Preliminary scientific report.
Optical nanoscopy reveals SARS-CoV-2-induced remodeling of human airway cells, Wilco Nijenhuis, Hugo G.J. Damstra, Emma J. van Grinsven, Malina K. Iwanski, Patrique Praest, Zahra E. Soltani, Mariëlle M.P. van Grinsven, Jesse E. Brunsveld, Theun de Kort, Lisa W. Rodenburg, Dorien C.M. de Jong, Henriette H.M. Raeven, Sacha Spelier, Gimano D. Amatngalim, Anna Akhmanova, Monique Nijhuis, Robert Jan Lebbink, Jeffrey M. Beekman, Lukas C. Kapitein, bioRxiv 2021.08.05.455126; doi: https://doi.org/10.1101/2021.08.05.455126, https://www.biorxiv.org/content/10.1101/2021.08.05.455126v1