In a study recently published on the medRxiv* preprint server, scientists from Singapore have assessed the levels of IgA-specific anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies in the breast milk of women recently immunized with the second dose of an mRNA-based coronavirus disease 2019 (COVID-19) vaccine. The findings reveal a lower titer of antibodies against SARS-CoV-2 Variants of Concern (VOCs) in the breast milk after vaccination.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Studies conducted in real-world situations have demonstrated that Pfizer/BioNTech-developed COVID-19 vaccine BNT162b2 can elicit anti-SARS-CoV-2 antibodies in human breast milk. However, it remains uncertain whether these antibodies can protect against emerging VOCs of SARS-CoV-2 and how long the protection persists. Since clinical studies testing vaccine efficacy did not involve pregnant or lactating women, more real-world studies are required to investigate the extent of passive immunity in infants.
In the current study, the scientists have investigated whether BNT162b2 vaccination can induce IgA-specific antibodies in breast milk against the spike receptor-binding domains (RBD) of SARS-CoV-2 VOCs. Specifically, they have targeted four VOCs, including alpha (first detected in the UK), beta (first detected in South Africa), gamma (first detected in Brazil), and delta (first detected in India) variants.
Study design
The study was conducted on 46 lactating women who received two doses of the Pfizer/BioNTech vaccine BNT162b2 at an interval of 21 days. Milk samples were collected before vaccination (baseline), 3 – 7 days after the second vaccination, and 4 – 6 weeks after the second vaccination. The levels of IgA-specific anti-RBD antibodies against the original SARS-CoV-2 strain and four VOCs were measure using enzyme-linked immunosorbent assay (ELISA).
Important observations
The study findings revealed that the antibodies could be detected in breast milk within 3 – 7 days after the second vaccination. In addition, the levels of antibodies developed against the beta, gamma, and delta variants were 28 – 33% lower than those developed against the original viral strain. However, no difference in antibody level was observed between the original SARS-CoV-2 and the alpha variant.
A reduction in antibody levels in the breast milk was observed after 4 – 6 weeks of the second vaccination. However, the levels were still higher than the pre-vaccination levels. Compared to the original SARS-CoV-2, a 25 – 30% reduction in antibody levels was observed for all tested VOCs except for the alpha variant.
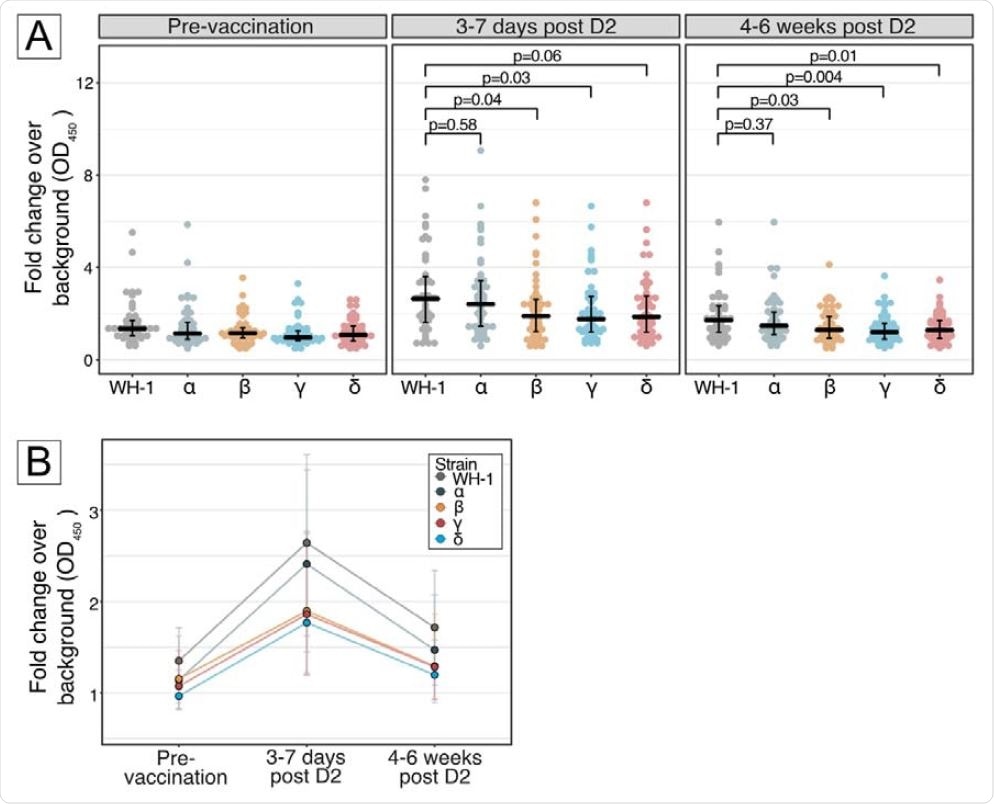
Panel A, Differential human milk IgA binding to receptor-binding domain (RBD) of SARS-CoV-2 spike protein of the ancestral Wuhan-Hu-1 strain (WH-1), Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1) and Delta (B.1.617.2) variants of concern, after vaccination with BNT162b2 across 3 time points: pre-vaccination, 3-7 days post D2 and 4-6 weeks post D2. Each dot denotes an individual sample. Centre line denotes the median, and error bars show Quartile 1 (bottom bar) and Quartile 3 (top bar). P values were calculated using Dunn’s Many-to-One Rank Comparison Test and reported for VOC compared against WH-1. At 3-7 days post D2, reduction from WH-1 for Alpha = 8%, Beta = 28%, Gamma = 33%, Delta = 30%. At 4-6 weeks post D2, reduction from WH-1 for Alpha = 14%, Beta = 25%, Gamma = 30%, Delta = 25%. Panel B, Kinetics of human milk IgA binding across 3 timepoints for ancestral Wuhan-Hu-1 strain (WH-1), Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1) and Delta (B.1.617.2) variants of concern. Each line represents 1 strain. Dots represent the median and error bars represent interquartile range.
Study significance
The study reveals that BNT162b2-induced IgA antibodies against SARS-CoV-2 and its variants remain detectable in breast milk six weeks after the second vaccination. This indicates that mucosal immunity against SARS-CoV-2 can be transferred from breastfeeding mothers to infants who are not eligible for vaccination and are at high risk of severe COVID-19.
However, the reduction in antibody levels with time observed in the study indicates a rapid waning of vaccine-induced immunity in infants.
As mentioned by the scientists, the study findings are limited to binding antibodies only. No experiment has been conducted to assess the virus-neutralizing ability of the antibodies. However, there is evidence suggesting that IgA binding antibodies in the breast milk of vaccinated women positively correlate with neutralization.
Overall, the study highlights the importance of vaccinating pregnant women to ensure the transfer of long-lasting passive immunity to infants via the placenta. Moreover, lactating mothers should be prioritized for vaccination to improve the level of protection in susceptible infants.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources