The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is the virus responsible for the ongoing coronavirus disease 2019 (COVID-19) pandemic, has already infected more than 233 million individuals and claimed more than 4.7 million lives across the world. SARS-CoV-2 is a ribonucleic acid (RNA) virus belonging to the coronaviridae family.
Scientists have developed several COVID-19 vaccines to manage this pandemic, many of which have already received emergency use authorization (EUA) from global regulatory bodies around the world. This has allowed vaccination programs to commence in many countries.
 Study: Antibody titers against the Alpha, Beta, Gamma, and Delta variants of SARS-CoV-2 induced by BNT162b2 vaccination measured using automated chemiluminescent enzyme immunoassay. Image Credit: anyaivanova / Shutterstock.com
Study: Antibody titers against the Alpha, Beta, Gamma, and Delta variants of SARS-CoV-2 induced by BNT162b2 vaccination measured using automated chemiluminescent enzyme immunoassay. Image Credit: anyaivanova / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The COVID-19 vaccines based on messenger RNA (mRNA) technology have been found to generate rapid and robust immunity as compared to the immune protection induced after natural infection. The BNT162b22 and mRNA-1273 vaccines, in particular, elicit high titers of SARS-CoV-2 neutralizing antibodies (NAbs) and are associated with high efficacy in preventing illness and reducing disease severity within the range of 94% and 95%.
It is important to measure NAbs to determine the status of protective immunity against viral infection. Currently, live SARS-CoV-2 and pseudotyped virus-based assays are widely used because of their safety and versatility. However, these assays require long readout times and are low throughput.
A new study published on the preprint server medRxiv* discusses the development of dedicated reagents against the SARS-CoV-2 spike protein through the use of an automated chemiluminescent enzyme immunoassay (CLEIA) system referred to as AIA-CL.
About the study
For this study, scientists analyzed samples from 168 Japanese healthcare workers who had received two doses of the BNT162b2 vaccine. They studied immunoglobulin G (IgG) index values using the automated CLEIA system AIA-CL and investigated the factors affecting the antibody titer. Subsequently, the spike protein (SP) IgG index was compared with 50% neutralization titers.
The AIA-CL method provided good index values of the antibody response after patients were vaccinated with the BNT162b2 vaccine. The system is a high throughput serological test capable of processing 120 samples per hour. AIA-CL is very robust and can concurrently detect IgGs against the nucleocapsid protein (NP) and SP of SARS-CoV-2.
The data obtained revealed that SP IgG index values in fully vaccinated subjects had an average correlation with neutralizing activities using lentivirus-based pseudovirus assays. Owing to the versatility of this method, it could be modified to evaluate subjects who have received different types of COVID-19 vaccines and also screen for herd immunity.
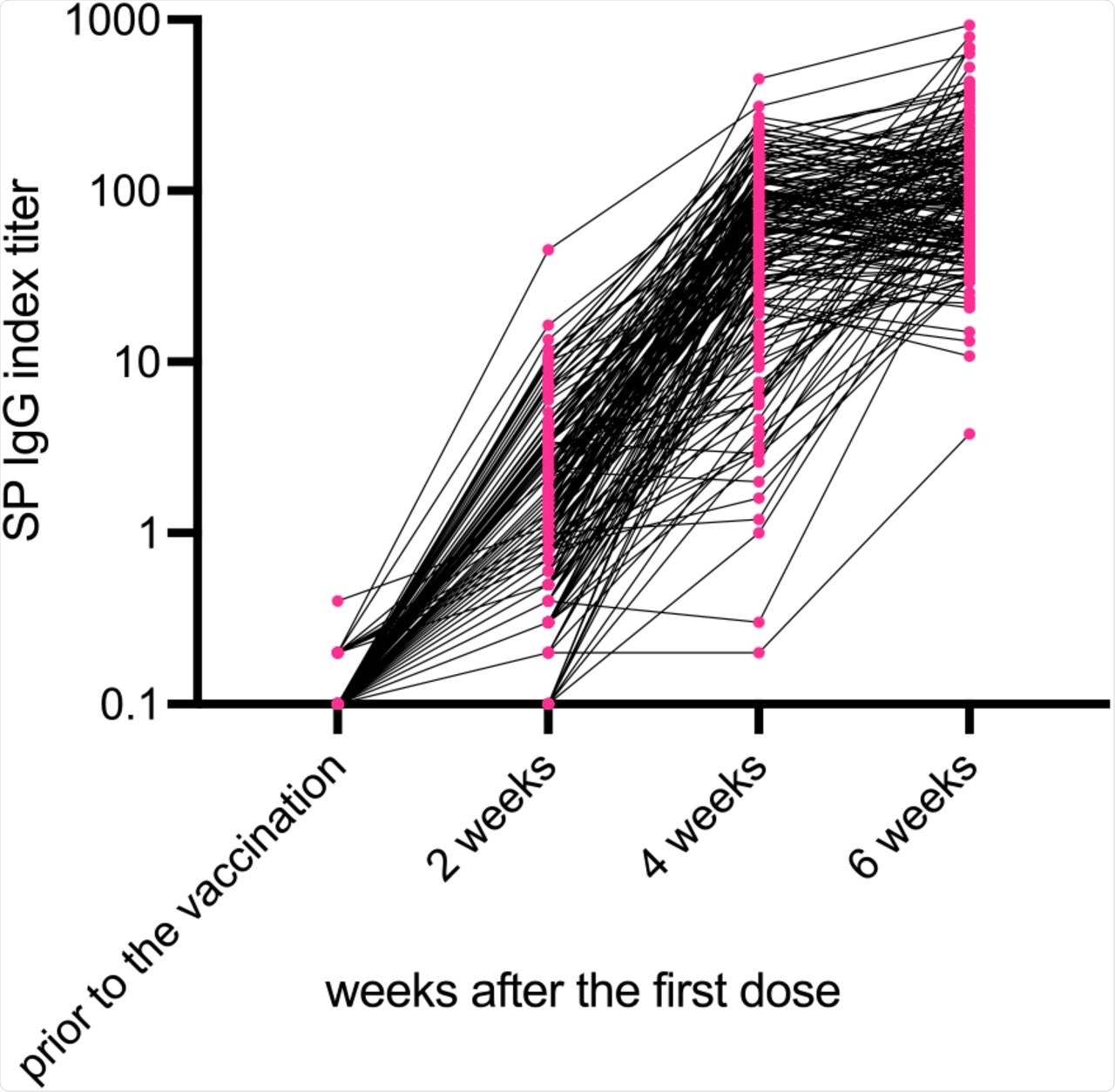 SP IgG titers were measured by the AIA-CL system before and then at 2, 4, and 6 weeks after the first dose in the subjects who underwent two doses of the BNT162b2 vaccine. Before vaccination, SP IgG titers of all subjects were below the threshold (index 1.0). SP IgG index titers had increased in 2, 4, and 6 weeks after the first dose. Abbreviations: SP, spike protein; IgG, immunoglobulin.
SP IgG titers were measured by the AIA-CL system before and then at 2, 4, and 6 weeks after the first dose in the subjects who underwent two doses of the BNT162b2 vaccine. Before vaccination, SP IgG titers of all subjects were below the threshold (index 1.0). SP IgG index titers had increased in 2, 4, and 6 weeks after the first dose. Abbreviations: SP, spike protein; IgG, immunoglobulin.
The vaccine studied here did not show sufficient humoral immunity after one dose. Rather, the BNT162b2 vaccine appeared to induce high immunity at three weeks after the second vaccination.
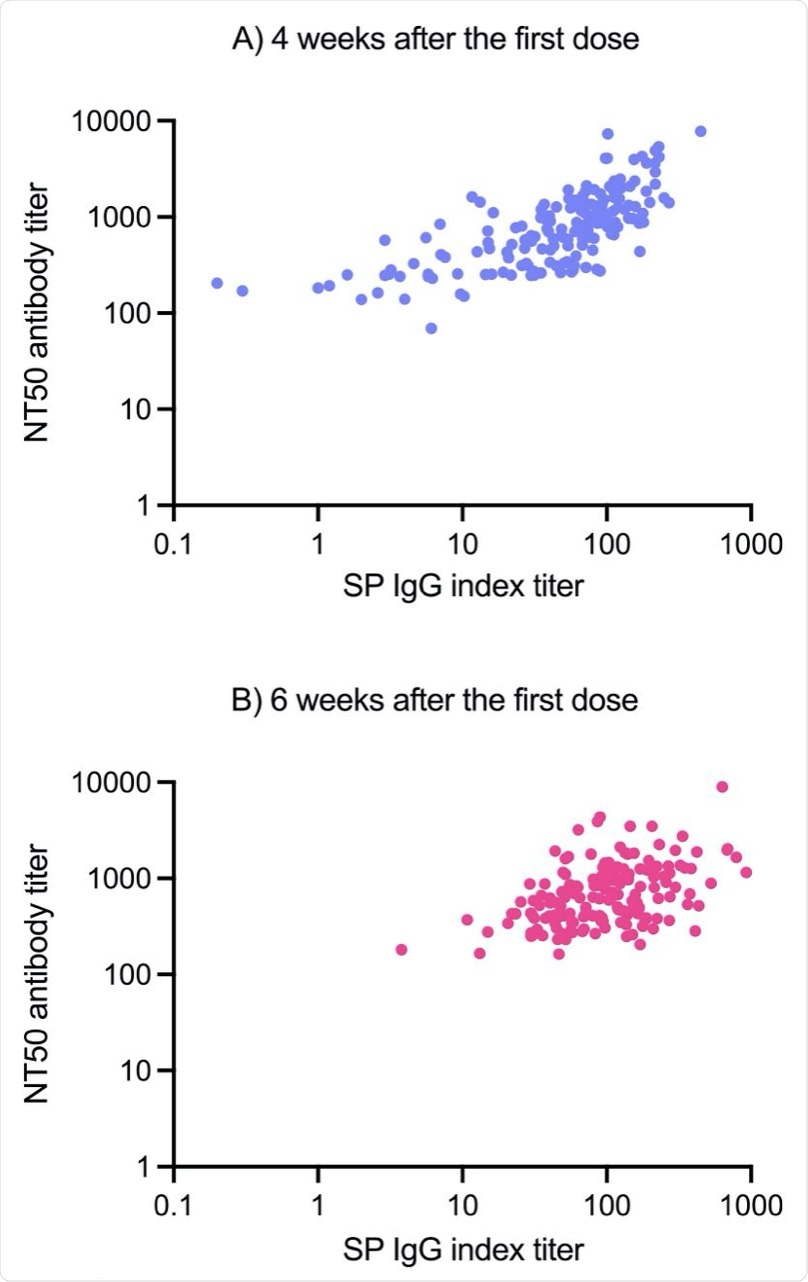 Correlation between NT50 neutralizing antibody titers and AIA-CL SP IgG titer at 4 (A) and 6 (B) weeks after the first dose in the subjects who underwent two doses of BNT162b2 vaccine.
Correlation between NT50 neutralizing antibody titers and AIA-CL SP IgG titer at 4 (A) and 6 (B) weeks after the first dose in the subjects who underwent two doses of BNT162b2 vaccine.
In the current context, vaccine breakthrough infections are rising and that is a cause for concern. In a related study, scientists had observed that NAb titers in individuals who developed breakthrough infections were substantially lower than those without breakthrough infections (177.7 vs. 501.3).
In the fully vaccinated individuals, researchers reported a NAb index of 100 or an AIA-CL SP IgG index of 10, which must be interpreted carefully to determine the exact protective antibody titer. Typically, higher NAb titers and increased values of SP IgG index are required to reduce the risk of developing SARS-CoV-2 infection.
The current study also determined differential humoral immune responses induced by the BNT162b2 vaccine against both the original SARS-CoV-2 strain, as well as its variants of concern using the prototype reagent panels.
The researchers reported the levels of the SP IgG index against the SARS-CoV-2 Beta variant to be 54% of those of the original strain. However, this was not the case for the Alpha strain, where the SP IgG index value was found to be double that of the original strain. This result was not in line with an earlier study associated with the Alpha strain, which stated that the antibody titer elicited after vaccination was similar to that of against the original strain.
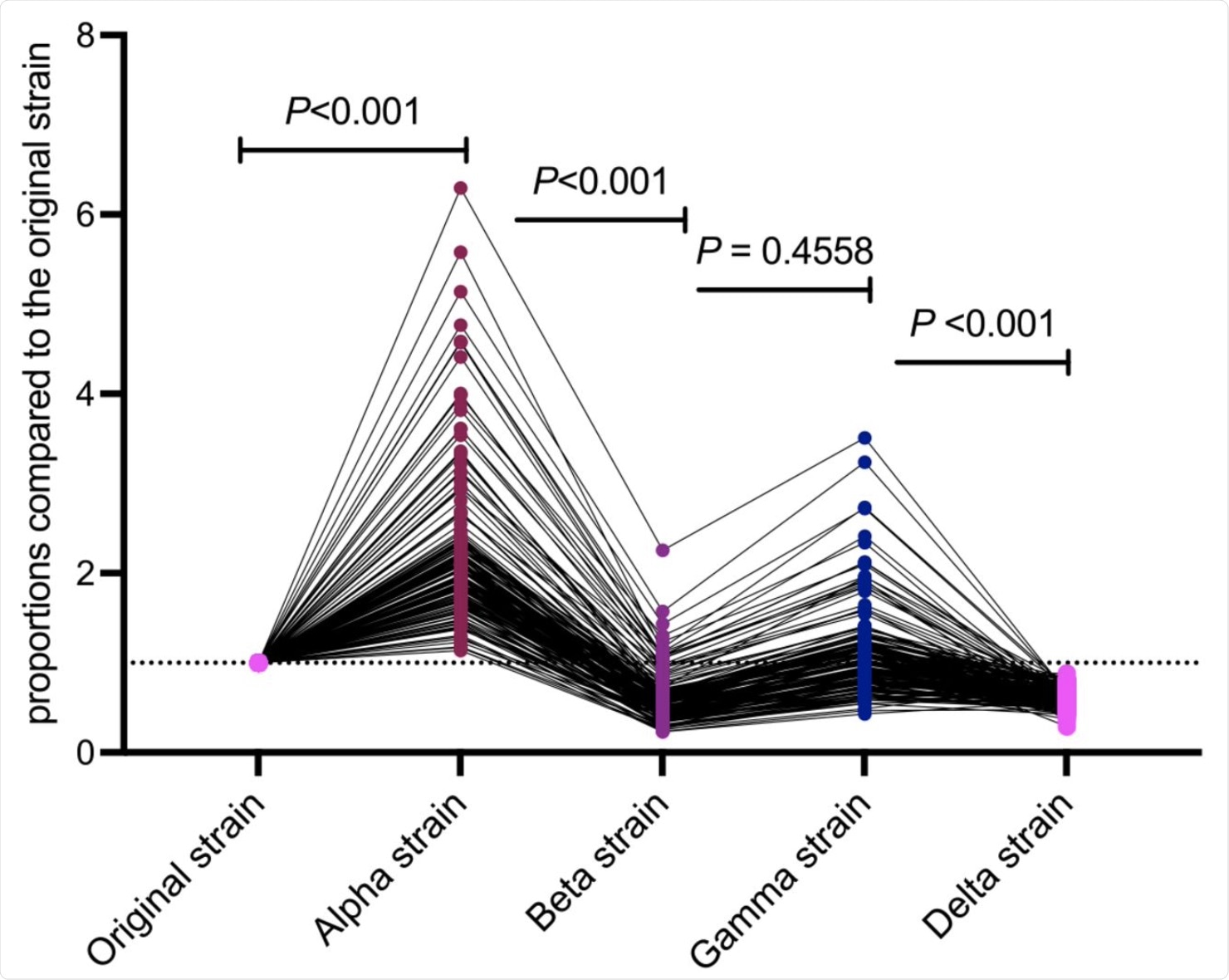 Proportions of antibody titers against the original, Alpha, Beta, Gamma, and Delta strains at 6 weeks after the first dose.
Proportions of antibody titers against the original, Alpha, Beta, Gamma, and Delta strains at 6 weeks after the first dose.
The authors of this study indicated that the CLEIA is extremely sensitive and detects the difference in humoral responses among the SARS-CoV-2 variants. Therefore, this method could be used as a mass screening method.
This study pointed out some of the important factors which affect the SP IgG index titers in fully vaccinated subjects. For instance, the elderly exhibit lower antibody response. Also, another Japanese study showed that individuals who drank alcohol frequently had lower antibody titers than those who did not.
The role of body mass index (BMI) on the NAb levels post-vaccination is not well understood. A recent study on severely obese individuals revealed that the antibody titers against the SP obtained via natural infection were higher compared to humoral immunity induced after vaccination.
Conclusion
This study had a few limitations, which included a small study cohort, that could influence the relationship between predisposing factors and antibody titers. Another limitation of this study was the lack of inclusion of cell-mediated immunity, as COVID-19 vaccines can elicit both cell-mediated and humoral immunity.
The authors strongly emphasized that regardless of an individual’s characteristics, completion of vaccination is extremely essential, particularly for individuals with no prior history of COVID-19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Kato, H., Miyakawa, K., Ohtake, N., et al. (2021) Antibody titers against the Alpha, Beta, Gamma, and Delta variants of SARS-CoV-2 induced by BNT162b2 vaccination measured using automated chemiluminescent enzyme immunoassay. medRxiv. doi:10.1101/2021.09.23.21263927. https://www.medrxiv.org/content/10.1101/2021.09.23.21263927v1
- Peer reviewed and published scientific report.
Kato, Hideaki, Kei Miyakawa, Norihisa Ohtake, Hirofumi Go, Yutaro Yamaoka, Satoshi Yajima, Tomoko Shimada, Atsushi Goto, Hideaki Nakajima, and Akihide Ryo. 2021. “Antibody Titers against the Alpha, Beta, Gamma, and Delta Variants of SARS-CoV-2 Induced by BNT162b2 Vaccination Measured Using Automated Chemiluminescent Enzyme Immunoassay.” Journal of Infection and Chemotherapy, November. https://doi.org/10.1016/j.jiac.2021.11.021. https://www.jiac-j.com/article/S1341-321X(21)00332-9/fulltext.