The COVID-19 pandemic led to the implementation of multiple non-pharmaceutical interventions by national and local governments. These included lockdowns, social distancing mandates, restrictions on national and international travel, and business/educational institution closures.
Due to the measures taken to prevent the spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), there has been a significant loss of human life, long-term ill-health, and economic recessions. Vaccines were touted to be the way out of this dilemma. Among the earliest vaccines to be developed and approved against SARS-CoV-2 included the AstraZeneca adenovirus vector (ChAdOx1 nCoV-19) vaccine AZD1222.
Earlier studies have shown the vaccine to be effective and safe, leading to its deployment in over a hundred countries. The current study reports the analysis results of a Phase III double-blind randomized controlled trial of the vaccine.
The aim of the current study was to identify the efficacy of the vaccine in preventing symptomatic COVID-19 confirmed by reverse-transcriptase–polymerase-chain-reaction (RT-PCR) testing. The trial included multiple high-risk groups, as well as those at risk of high exposure to the virus.
Study findings
The current study included over 32,000 individuals who were randomized to a two-dose vaccine or placebo regimen. Vaccine and placebo doses were given intramuscularly, with twice as many subjects in the vaccine cohort. About a quarter of the participants were 65 years or older.
All individuals who received one or more doses of the vaccine were also assessed for vaccine safety. Efficacy analysis was confined to infection-naïve individuals by RT-PCR and antibody testing who were vaccinated with two doses and monitored for 15 or more days from the second dose.
The sample was diverse, with about 56% being male, and about 60% with one or more underlying conditions. The mean age was 50 years.
Almost 80% were White, with the remainder of the sample consisting of individuals of Black, Asian, and indigenous origins. Over 20% were Hispanic or Latinx. Less than 2% in either group had human immunodeficiency virus (HIV) infection under good control.
Adverse events occurred in 41% and 30% of the vaccine and placebo groups, respectively. While about 8% and 2% reported pain within 28 days of either dose, headache was reported by 6% and 5% in the vaccine and placebo groups, respectively.
A serious adverse event (AE) occurred within 28 days of a dose in 0.5% of participants in either group. This included death as a result of AEs in seven participants in each group. However, the researchers confirmed that these deaths were not related to the vaccine or placebo.
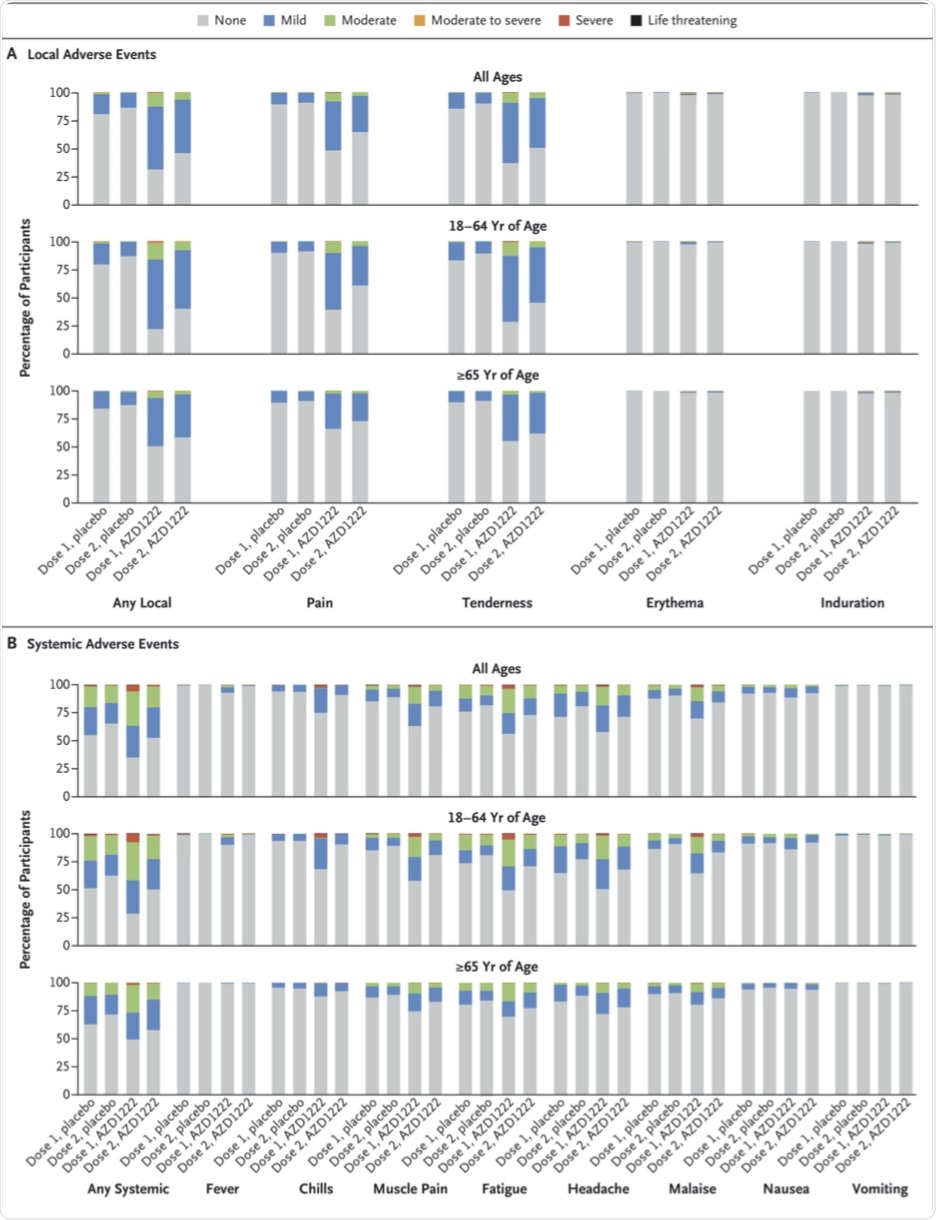 Local and Systemic Solicited Adverse Events after First and Second Dose, by Age Group.
Local and Systemic Solicited Adverse Events after First and Second Dose, by Age Group.
Two deaths occurred as a result of COVID-19 in the placebo group, but none in the vaccine group.
AEs potentially caused by immune reactions were similar in both groups, as were neurologic, vascular, and hematologic reactions. Particularly relevant in the face of currently circulating rumors on the vaccine is the observation of similar incidences of deep vein thrombosis, pulmonary embolism, a drop in platelet counts, and immune thrombocytopenia, at less than 0.1% in both groups.
There were no reported cases of thrombosis with thrombocytopenia, cerebral venous sinus thrombosis, or venous thrombosis. Furthermore, most local and systemic AEs resolved within two days of onset.
Efficacy-wise, the vaccine prevented symptomatic COVID-19. There were 73 cases in the vaccinated group, or 0.4% of the total, compared to 130 in the placebo group, or 1.5%. Taken together, vaccine efficacy was thus estimated to be 74%. The protective effect began soon after the second dose.
In individuals between the ages of 18-64 years, about 73% of symptomatic infections were prevented. This was comparable to 84% in those aged 65 years or above, independent of racial and ethnic differences, underlying illnesses, sex, or prior infection with the virus.
No cases of severe or critical COVID-19 occurred in the vaccinated group; however, there were eight cases in the placebo group. The vaccine prevented 95% of emergency visits related to COVID-19 and 94% of hospitalizations.
Vaccine efficacy against infection with SARS-CoV-2 was 64%, as assessed by all participants with positive anti-nucleocapsid antibodies since this antigen is not present in the vaccine. Neutralizing antibodies against SARS-CoV-2 were increased in the vaccine group, peaking after the second dose, as compared to consistently low titers in the placebo group.
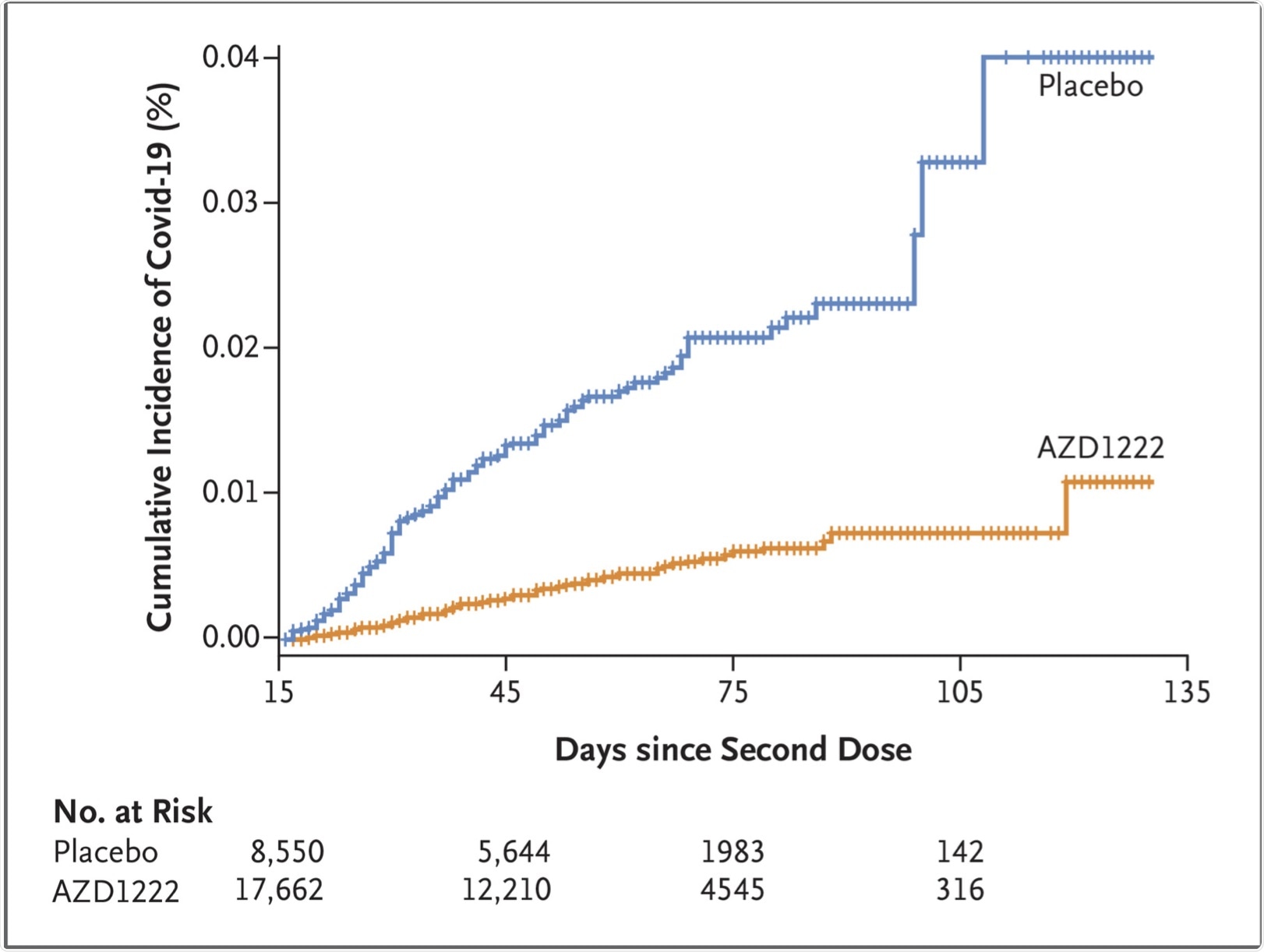 Time to First SARS-CoV-2 RT-PCR–Positive Symptomatic Illness Occurring 15 Days or More after the Second Dose (Fully Vaccinated Analysis Population).
Time to First SARS-CoV-2 RT-PCR–Positive Symptomatic Illness Occurring 15 Days or More after the Second Dose (Fully Vaccinated Analysis Population).
Implications
The AZD1222 vaccine appears to be safe and effective in preventing symptomatic COVID-19 among both young and older individuals. Two doses given at an interval of four weeks reduced symptomatic illness by 74% from 15 days after the second dose. Severe and critical cases were avoided altogether, while emergency room visits and hospitalizations were markedly reduced.
Significantly, the protective efficacy begins, though at a lower level, after a single dose. This finding was corroborated by the United Kingdom experience, where vaccine efficacy was demonstrated during the delayed second dose phase.
Safety data showed no increased risk of neurologic events, especially demyelinating disease or acute transverse myelitis, as compared with the placebo group. Antibody-dependent enhancement of respiratory symptoms was also reduced.
The overall incidence of thrombosis with or without accompanying thrombocytopenia was not increased in the vaccine group.
“Independent safety reviews by regulatory authorities of available clinical and real-world evidence have concluded that the benefits of AZD1222 outweigh the potential risks, with protection from the serious consequences of Covid-19 increasing with age and SARS-CoV-2 infection rate.”