The coronavirus disease 2019 (COVID-19) pandemic, which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to a high rate of respiratory-related mortality. Apart from pulmonary diseases, SARS-CoV-2 could also bring about injury to the liver, heart, kidney, disseminated intravascular coagulation, chronic fatigue, and rhabdomyolysis.
The variability of clinical outcomes of COVID-19 is due to sites of virus and viral antigen localization, the level of inflammation, vascular complications associated with the infection, host factors including genetics, age, gender, as well as the coexistence of other comorbid conditions. Additionally, the host’s immune response also has an impact on disease severity and outcome. For example, the type I interferon (IFN) pathway is a major immune pathway that can limit SARS-CoV-2 infection.
 Study: Autoantibodies Detected in MIS-C Patients due to Administration of Intravenous Immunoglobulin. Image Credit: createiveneko / Shutterstock.com
Study: Autoantibodies Detected in MIS-C Patients due to Administration of Intravenous Immunoglobulin. Image Credit: createiveneko / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
What is MIS-C?
Most children and young adolescents report either asymptomatic or mild cases of COVID-19. However, a rare and severe complication of COVID-19 has been detected in children between four and six weeks after the initial infection. This condition is known as a multisystem inflammatory syndrome in children (MIS-C) and is associated with high fever, rash, multi-organ dysfunction, and elevated markers of inflammation.
Furthermore, the clinical manifestations of MIS-C resemble Kawasaki Disease (KD), which is a vasculitis that typically affects children. Gastrointestinal symptoms and myocardial involvement are commonly seen in children with MIS-C. MIS-C and KD can be treated by high-dose intravenous immunoglobulin (IVIG), corticosteroids, and biologic agents.
Since the onset of the COVID-19 pandemic, efforts are being made to understand the differences in symptoms and complications seen in COVID-19 patients. Several studies have shown the importance of the immune system in controlling infection, as well as preventing tissue damage.
Recent studies have identified autoantibodies in the serum samples of adults and children with COVID-19 and children with MIS-C. However, the role of high-dose IVIG administration in detecting these autoantibodies has not been studied yet.
A new study published on the preprint server medRxiv* assessed the presence of autoantibodies against a panel of autoantigens associated with known autoimmune diseases in both adults and children with COVID-19.
About the study
The current study involved the collection of serum samples from patients infected with COVID-19, healthy control individuals, and children with MIS-C or KD. The samples were collected from multiple geographical sites that included the United States, Italy, Chile, and Israel.
The Luciferase Immunoprecipitation Systems (LIPS) assay was conducted for the detection of autoantibodies and to determine the levels of autoantibodies in three different batches of IVIG.
Study findings
The study results indicated that children with MIS-C had a median age of six years, while children with COVID-19 had a median age of one year. An equal number of males and females were reported among MIS-C patients, while male dominance was found in patients with COVID-19.
Most of the patients were Hispanic/Latino, followed by Caucasians, and a few were Black. The most common symptom reported from the MIS-C cohort was fever followed by diarrhea, vomiting, and abdominal pain.
The analysis of adult patients with COVID-19 found high levels of autoantibodies against several autoantigens such as the lung protein KCNRG, gastric ATPase, and systemic lupus erythematosus (SLE) antigen Sm-D3. In addition, several other autoantigens such as Ro52, Ro60, La, and GAD65 were detected at lower levels.
Some adults showed elevated levels of SmD3, as well as Ro52, Ro60, and La. However, the levels of both KCNRG and Sm-D3 autoantibodies were found to be high in patients with severe or moderate COVID-19 but not in critically ill patients.
The results further indicated that children with COVID-19 reported little or no autoantibodies against KCNRG, Sm-D3, and the other autoantigens that were tested. However, one child reported high levels of GAD65 autoantibodies, which is a well-known marker of type 1 diabetes. The child presented ketoacidosis and required insulin upon hospital admission. Additionally, SARS-CoV-2 infection was found to induce both autoantibody-positive and autoantibody-negative diabetes, as well as trigger autoimmune diseases.
The results also showed that children with MIS-C lacked La, Ro60, and gastric ATPase autoantibodies. Autoantibodies against Ro52, Ro60, La, and gastric ATPase were detected in MIS-C and KD children only upon IVIG administration. The presence of seropositive autoantibodies in IVIG depends on the donor pools that include asymptomatic or pre-symptomatic individuals.
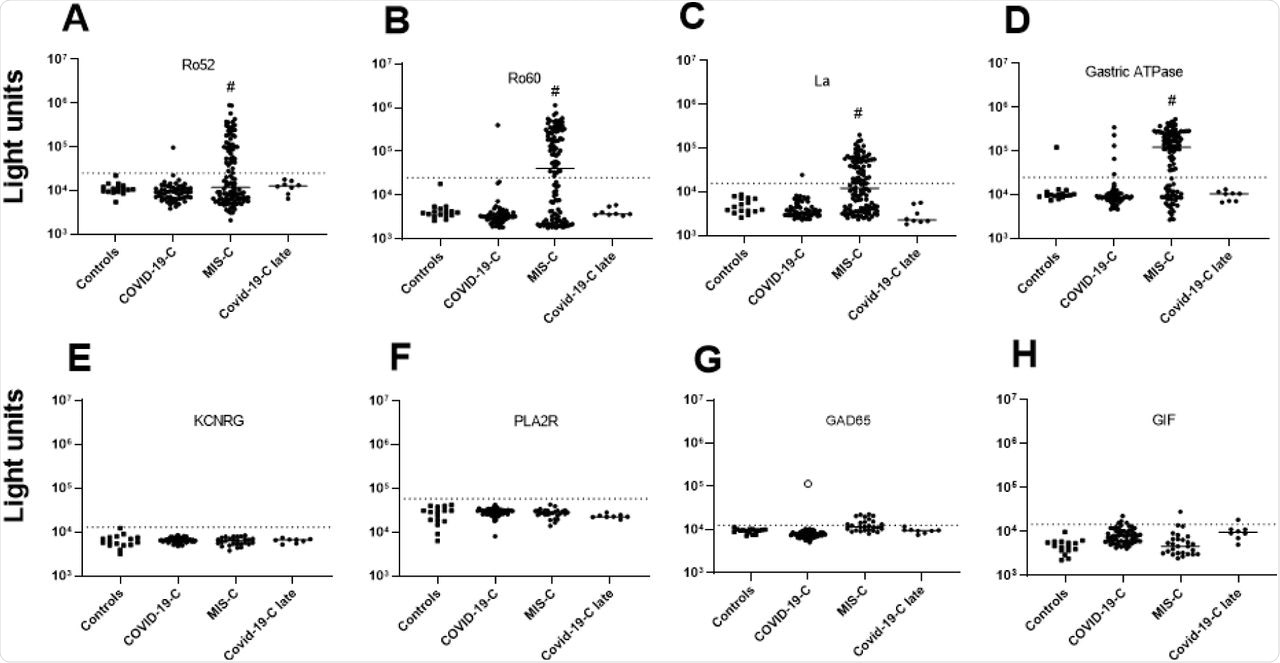 (A-D) Autoantibody levels against Ro52, Ro60, La, and gastric ATPase were determined in 16 children not infected with SARS-CoV-2 (Controls), 59 children with COVID-19 (COVID-19-C), 54 children with MIS-C, and 8 children who recovered from acute COVID-19 (COVID-19-C late). Each symbol represents a sample from an individual patient or different time points from an individual patient (E-F) Autoantibody levels against four autoantigens, KCNRG, PLA2R, GAD65, and GIF were tested in the same children as in panels A-D, except for MIS-C children where a single sample from the time of peak Ro52, Ro60, La, and/or gastric autoantibodies was used. Autoantibody levels are plotted on the Y-axis in light units on a log10 scale. The dashed lines represent the cutoff levels for determining positive autoantibody levels for each target antigen as described in the Methods. The sample from the child with acute COVID-19 with high levels of GAD65 autoantibodies is denoted by the open black circle. Statistically significant higher autoantibody levels seen in MIS-C patients treated with IVIG compared to the other groups are shown by the hash tag.
(A-D) Autoantibody levels against Ro52, Ro60, La, and gastric ATPase were determined in 16 children not infected with SARS-CoV-2 (Controls), 59 children with COVID-19 (COVID-19-C), 54 children with MIS-C, and 8 children who recovered from acute COVID-19 (COVID-19-C late). Each symbol represents a sample from an individual patient or different time points from an individual patient (E-F) Autoantibody levels against four autoantigens, KCNRG, PLA2R, GAD65, and GIF were tested in the same children as in panels A-D, except for MIS-C children where a single sample from the time of peak Ro52, Ro60, La, and/or gastric autoantibodies was used. Autoantibody levels are plotted on the Y-axis in light units on a log10 scale. The dashed lines represent the cutoff levels for determining positive autoantibody levels for each target antigen as described in the Methods. The sample from the child with acute COVID-19 with high levels of GAD65 autoantibodies is denoted by the open black circle. Statistically significant higher autoantibody levels seen in MIS-C patients treated with IVIG compared to the other groups are shown by the hash tag.
Additionally, the in vivo decay of these autoantibodies was studied. The analysis of MIS-C IVIG recipients indicated that Ro52, Ro60, and La autoantibodies showed a similar decay profile where they persisted in the blood for several weeks before becoming undetectable by 35-60 days. However, the autoantibodies against gastric ATPase were found to persist for a longer duration.
Therefore, it can be concluded that the current study did not detect the presence of any autoantibodies against known autoimmune diseases in children with MIS-C. However, since only limited autoantigens were considered in the study, other autoantibodies with MIS-C can exist.
Taken together, further studies are required to determine whether specific autoantibodies play a role in the immune complex formation or autoantibody-driven damage to host cells in children with MIS-C.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Burbelo, P. D., Castagnoli, R., Shimizu, C., et al. (2021). Autoantibodies Detected in MIS-C Patients due to Administration of Intravenous Immunoglobulin. medRxiv. doi:10.1101/2021.11.03.21265769. https://www.medrxiv.org/content/10.1101/2021.11.03.21265769v1.
- Peer reviewed and published scientific report.
Burbelo, Peter D., Riccardo Castagnoli, Chisato Shimizu, Ottavia M. Delmonte, Kerry Dobbs, Valentina Discepolo, Andrea Lo Vecchio, et al. 2022. “Autoantibodies against Proteins Previously Associated with Autoimmunity in Adult and Pediatric Patients with COVID-19 and Children with MIS-C.” Frontiers in Immunology 13 (March). https://doi.org/10.3389/fimmu.2022.841126. https://www.frontiersin.org/articles/10.3389/fimmu.2022.841126/full.