In their work, the researchers found that the quality of available rapid tests is comparable to the currently used major commercial laboratory testing kits. Based on diminishing efficacy with time, the researchers recommend personalized booster programs to reduce breakthrough infections and to optimize booster dose supplies as opposed to a booster program at specific post-vaccination time points.
 Study: Individual vaccine efficacy variation with time since mRNA BNT162b2 vaccination estimated by rapid, quantitative antibody measurements from a finger-prick sample. Image Credit: Cryptographer / Shutterstock.com
Study: Individual vaccine efficacy variation with time since mRNA BNT162b2 vaccination estimated by rapid, quantitative antibody measurements from a finger-prick sample. Image Credit: Cryptographer / Shutterstock.com

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
About the study
In the current study, the researchers present a high-sensitivity analytical assay for estimating the antibody concentration from blood prick samples. After the whole blood sample is collected for quantitative immunity measurements, all operations, including loading the sample onto the measurement instrument, are automated on the Orbis high-throughput quantitative immunity measurement system.
This system is a centrifugal microfluidic platform that is based on an enzyme-linked immunosorbent assay (ELISA) and has been adapted for accurate, precise, and rapid testing. Important elements of the assay design include its rapid capabilities of completing the experiment in less than 20 minutes, complete mixing, and accurate timing for a kinetically controlled assay.
The analyte concentration is deduced from the rate of binding of the target to the capture surface, where a wide and linear dynamic range is achieved. A small bead is used to capture the analyte, which is the antibody immunoglobulin G (IgG), on its surface that carries the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) receptor-binding domain (RBD) protein.
For the measurement of the IgG concentration, mixing is key. This is achieved by the rapid oscillation of the disc motion, which causes oscillation of the beads within the chamber. The shape of the chamber is also controlled to promote mixing. The samples are diluted with 10x addition of buffer, which also minimizes the effects of non-specific adsorption of blood components.
Study findings
In the current study, participants within the age range of 20 to 60 years were vaccinated with a range of age and time since completion of vaccination, as defined as two doses separated by at least three weeks. All participants in the current study were vaccinated against the coronavirus disease 2019 (COVID-19) with the Pfizer-BioNTech BNT162b2 vaccine.
Comparison results showed that there was a significant scatter of results across the different platforms, thereby indicating that the Orbis assay results are very comparable with many commercial assay platforms. The researchers demonstrated repeatability of duplicate measurements of 10% across a range of 5-500 ng/mL of IgG.
To demonstrate the system’s ability to distinguish fully vaccinated from unvaccinated people, the researchers conducted a small clinical trial. The resulting data from the study provided a distribution across the anti-SARS-CoV-2 RBD IgG concentration that was analyzed by researchers to determine the change in the antibody concentration distribution with time since vaccination.
As compared to the unvaccinated individuals, who had a signal that was significantly smaller and normally distributed, the results for vaccinated participants showed a log-normal distribution of concentration, which indicates a reduction in time since vaccination.
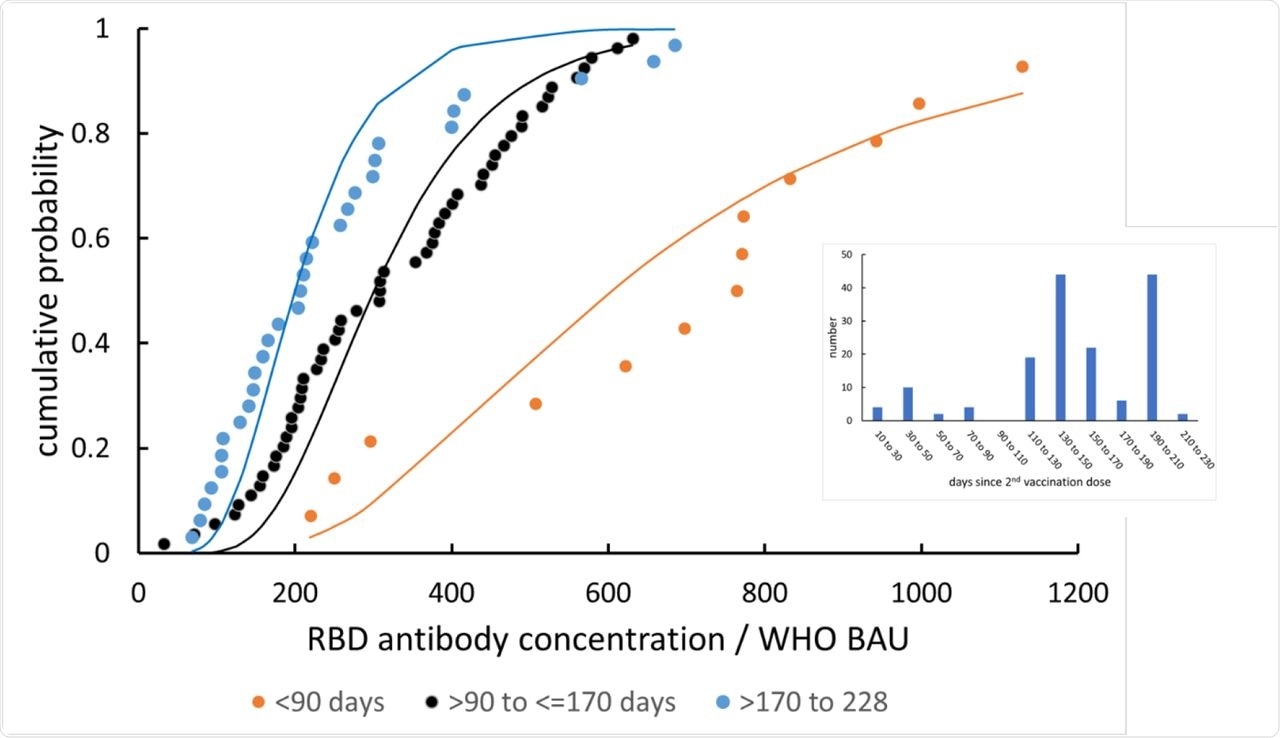 Anti-RBD IgG concentration distribution (offset subtracted), and its alteration with time since vaccination. The lines are fits to a log-normal distribution. Inset: distribution of vaccination dates across the study participants.
Anti-RBD IgG concentration distribution (offset subtracted), and its alteration with time since vaccination. The lines are fits to a log-normal distribution. Inset: distribution of vaccination dates across the study participants.
“The effect of the log-normal distribution of concentration is that the most significant contribution to breakthrough infections comes from people in the low-concentration tail of the distribution.”
The researchers found that the small signal in the unvaccinated people was a result of non-specific adsorption of the secondary indicator antibody promoted by fibrinogen adsorption to the assay chamber walls. Therefore, they treated this result as a normally distributed offset error.
The researchers also listed the parameters and compared the observed and calculated population vaccine efficacy. To this end, vaccination date correlated data fell into three distinguishable ranges of less than 90 days, 90-170 days, and 170-228 days.
Population vaccine efficacy was found to as highly significant between less than 90 days and 90-170 days, and marginally significant between 90-170 days and 170-228 days. More specifically, the researchers observed the estimated median vaccine efficacy to be between 65-95% at less than 90 days post-vaccination, and between 35-90% at both 90-170 days and 170-230 days, with an average efficacy of 70% and 65%, respectively. The implications of these findings are to direct booster vaccination programs at optimizing the use of booster doses to achieve the best possible population protection.
Conclusions
Despite the limitations of this small study, without comprehensive coverage of the full range of vaccination dates, the effect of time since vaccination on individual vaccine efficacy distribution is presented. Furthermore, the model used in the study has captured the general trend well.
The expected primary outcome through this simple and rapid procedure might, for example, be used for the entry control or validation of a vaccine passport, which is of immense current interest in the wake of the ongoing COVID-19 pandemic.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.