
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The emergence of the new SARS-CoV-2 Omicron variant of concern (VOC), which was quickly followed by a staggering surge in coronavirus disease 2019 (COVID-19) cases across the globe, is alarming. Researchers have discovered over 35 mutations in the spike (S) protein of the B1.1.529 variant that may confer increased transmissibility and immune-escape abilities to this new strain of SARS-CoV-2.
Several studies have reported decreased neutralizing potency of sera from vaccinated individuals and convalescent patients; therefore, new investigations to determine cellular immunity against the Omicron variant are essential.
About the study
In the present study, researchers examined the sera samples of vaccinated individuals to investigate T-cell reactivity against the mutated regions in the S protein of the SARS-CoV-2 Omicron variant, as well as the remaining T-cell immunity against the whole S protein by peptide stimulation analysis.
A total of 61 vaccinated individuals were selected for the study, which included hospital workers, scientists, and their family members or acquaintances. The participants were classified into five groups based on vaccination status and COVID-19 history: 1) two-dose vaccine recipients, 2) three-dose vaccinees, 3) heterologous vaccinees (adenoviral vaccine + messenger ribonucleic acid [mRNA] vaccine), 4) vaccinated individuals with subsequent SARS-CoV-2 infection, and 5) infected individuals who were subsequently vaccinated.
Peripheral blood mononuclear cells (PBMCs) were isolated from the blood samples of participants and an in vitro assay was performed with three different peptide pools of 15 amino acids length. One of the peptide pools was constructed with the peptides of the whole S protein of the original SARS-CoV-2 strain. The second pool (PoolMut) had only the mutated regions of the Omicron variant, whereas the third pool (PoolRef) consisted of both the Omicron and original SARS-CoV-2 strain regions.
Study findings
The frequency of donors who showed CD4+ and CD8+ T-cell reactivity against PoolS was observed to be 100% and 96%, respectively. The CD4+ and CD8+ T-cell reactivity was detected in 74% and 60% donors, respectively, against PoolMut of the Omicron variant. Further, 96% and 63% donors, respectively, reported CD4+ and CD8+ T-cell reactivity against PoolRef.
Heterologous vaccinees reported CD4+ (91%) and CD8+ (73%) reactivity to the PoolMut. Comapratively, participants who received two vaccine doses reported T-cell reactivity of 70% and 30% for CD4+ and CD8+, respectively, to the PoolMut.
The study observed a lower fraction of CD4+ and CD8+ T-cells in response to the mutated regions of the Omicron S protein. The reduction of T-cell reactivity to the mutated regions of the Omicron variant was significant across all five groups of participants, irrespective of vaccination and COVID-19 history. Further, the findings reported that interferon-γ (IFN- γ) release was also low when incubated with mutated regions of the Omicron S protein (PoolMut) as compared to that with PoolRef.
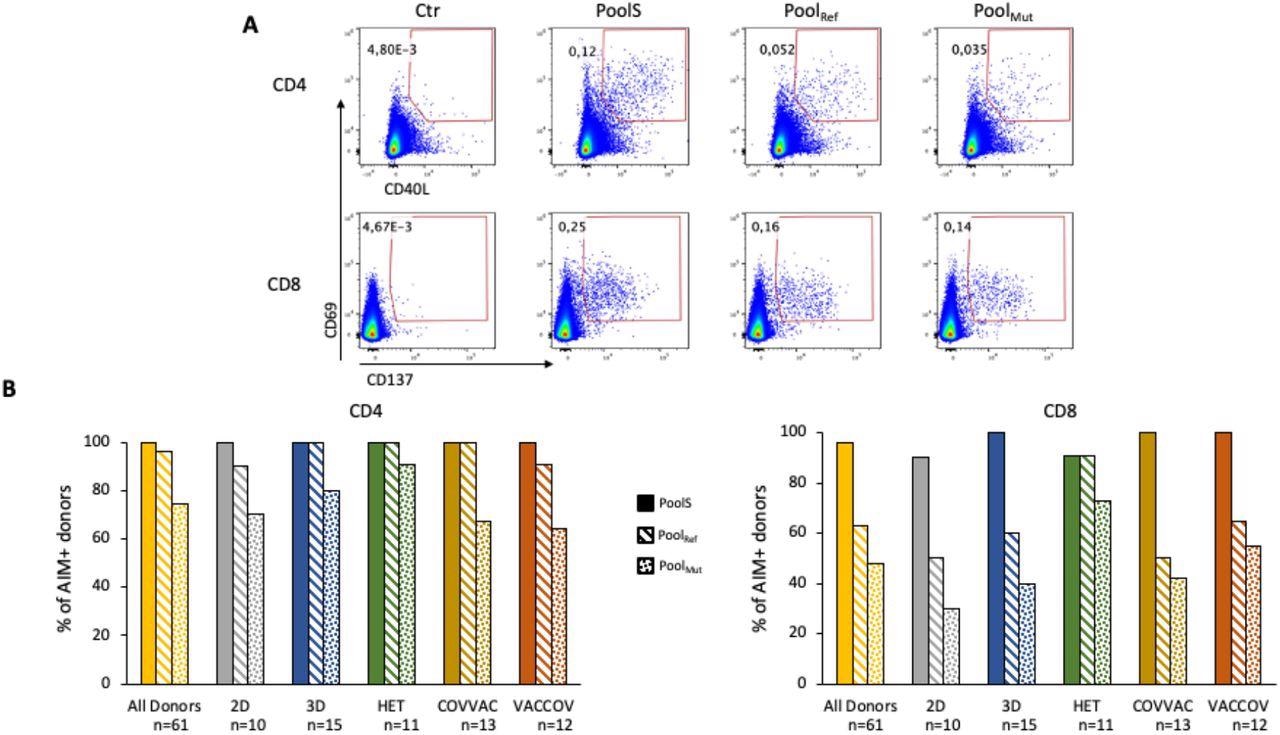
T cell responses to the Spike protein. A) Representative flow cytometry plots gated on CD4+ or CD8+ T cells showing up-regulation of activation markers (CD69 and CD40L for CD4+ cells and CD69 and CD137 for CD8 cells) following overnight stimulation with a pool of overlapping peptides covering the whole Spike protein from the ancestral vaccine strain (Pools), a peptide pool covering only the mutated regions of the Spike protein from the Omicron variant (PoolMut) or a peptide pool covering the same regions as above, but from the ancestral strain (PoolRef). B) percentage of individuals in each group presenting CD4+ (left) and CD8+ (right) Spike-specific responses.
Conclusions
The study findings present evidence of a reduction in T-cell reactivity to the Omicron variant, which is particularly affected by mutations in the S protein of this variant. Notably, the decrease in reactivity of T-cells was observed against a small fraction of total protein, and the authors reported the overall T-cell reactivity to S protein of the new mutant to be 83%.
Cellular immunity to the whole S protein pool was noted in 100% of donors. However, the mutations in the S protein of the Omicron variant reduced T-cell responses by 47%. Taken together, the findings of the current study showed that the T-cell response to the Omicron variant is maintained, regardless of COVID-19 history or vaccination, and may confer protection from severe illness.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
De Marco, L., D’Orso, S., Pirronello, M., et al. (2021). Preserved T cell reactivity to the SARS-CoV-2 Omicron variant indicates continued protection in vaccinated individuals. bioRxiv. doi:10.1101/2021.12.30.474453. https://www.biorxiv.org/content/10.1101/2021.12.30.474453v1.
- Peer reviewed and published scientific report.
De Marco, Lorenzo, Silvia D’Orso, Marta Pirronello, Alice Verdiani, Andrea Termine, Carlo Fabrizio, Alessia Capone, et al. 2022. “Assessment of T-Cell Reactivity to the SARS-CoV-2 Omicron Variant by Immunized Individuals.” JAMA Network Open 5 (4): e2210871. https://doi.org/10.1001/jamanetworkopen.2022.10871. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2791449.