A recent study suggests neutralizing antibody responses against variants of concern, including Omicron, differs significantly between vaccinated and naturally infected individuals. The research, posted to the medRxiv* preprint server, focused on vaccinated individuals who are pregnant — an understudied group in COVID-19 research.
Vaccinated individuals with a booster showed a robust and wide-ranging antibody response against Beta, Delta, and Omicron. While boosted individuals were protected against the variant, Omicron showed the most resistance to neutralization.
Omicron was identified through genomic surveillance efforts by South African scientists in November 2021 and is associated with mutations that evade the immune response.
The research team observed a reduced immune response in vaccinated pregnant individuals compared to vaccinated non-pregnant individuals. This suggests vaccinated pregnant women remain at a slightly higher risk of infection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern.
“Understanding optimal timing during pregnancy for booster doses will be important for protecting this population,” wrote the research team.
How they did it
The researchers studied donated convalescent plasma or serum samples from three cohorts: people with antibodies after recovering from a SARS-CoV-2 infection, vaccinated healthcare workers boosted with the Pfizer-BioNTech vaccine, and pregnant individuals immunized with two doses of an mRNA vaccine.
About 54 plasma samples from people with naturally acquired immunity were taken during the peak phase of their mild COVID-19 infection (28 days post-enrollment) and two other time points at days 210 and 300 after study enrollment.
These individuals were infected during the first half of 2020 when the dominant and circulating strain was SARS-CoV-2 with D614.
Naturally acquired immune responses against SARS-CoV-2 variants
Neutralizing antibody titers were at their peak on day 28 against all SARS-CoV-2 variants of concern. When the researchers looked at antibody titers on day 210 and day 300, they found antibody levels decreased over time.
The Beta and Omicron variant showed the most resistance to neutralization compared to the original strain and Delta when neutralizing antibody titers were at their highest. The researchers suggest this resistance may stem from shared mutations between Beta and Omicron that promote antibody escape.
By day 300, neutralizing activity against Beta and Omicron was largely nonexistent.
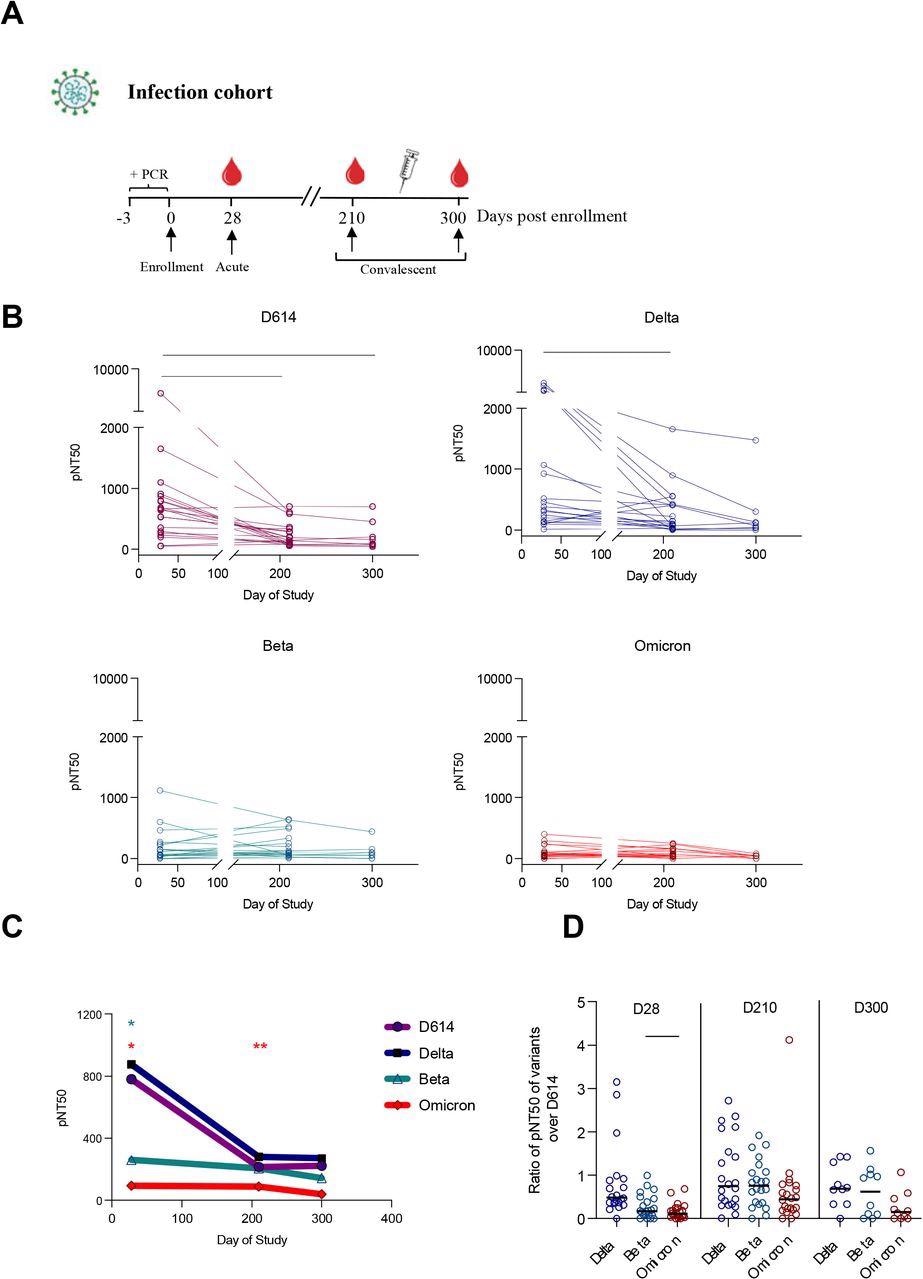
Plasma neutralizing titers against variants of concern in an outpatient COVID-19 infection cohort. (A) Study subjects were enrolled (day 0) within three days of a positive SARS-CoV-2 PCR test. Longitudinal plasma samples at an early study time-point (day 28, n=23), and two convalescent time points (day 210, n=23 and day 300, n=8) were assessed for neutralizing antibody against 4 SARS-CoV-2 pseudovirus strains: D614, Delta (B.1.617.2), Beta (B.1.351) and Omicron (B.1.1.529). (B) The kinetics of half-maximal SARS-CoV-2 pseudovirus neutralizing titers (pNT50) over time against D614, Delta (B.1.617.2), Beta (B.1.351) and Omicron (B.1.1.529). (C) Mean pNT50 values across study time-points of all 4 pseudovirus variants. Values significantly different from D614 at each time point are shown. (D) The ratio of pNT50 values of the variants of the concern Delta, Beta and Omicron over D614 pNT50 at each study time point. p values in (B-D) were calculated using mixed-effects analysis with Geisser-Greenhouse correction and Tukey’s multiple comparisons test.
Antibody response in vaccination pregnant individuals
The research team measured antibody responses from nine unvaccinated pregnant individuals when enrolling in the study and 33 who received two doses of an mRNA vaccine.
Serum samples collected after vaccination showed significant neutralizing activity against D614. While vaccines provided considerable protection against SARS-CoV-2 variants, antibody responses were much lower compared to the original strain.
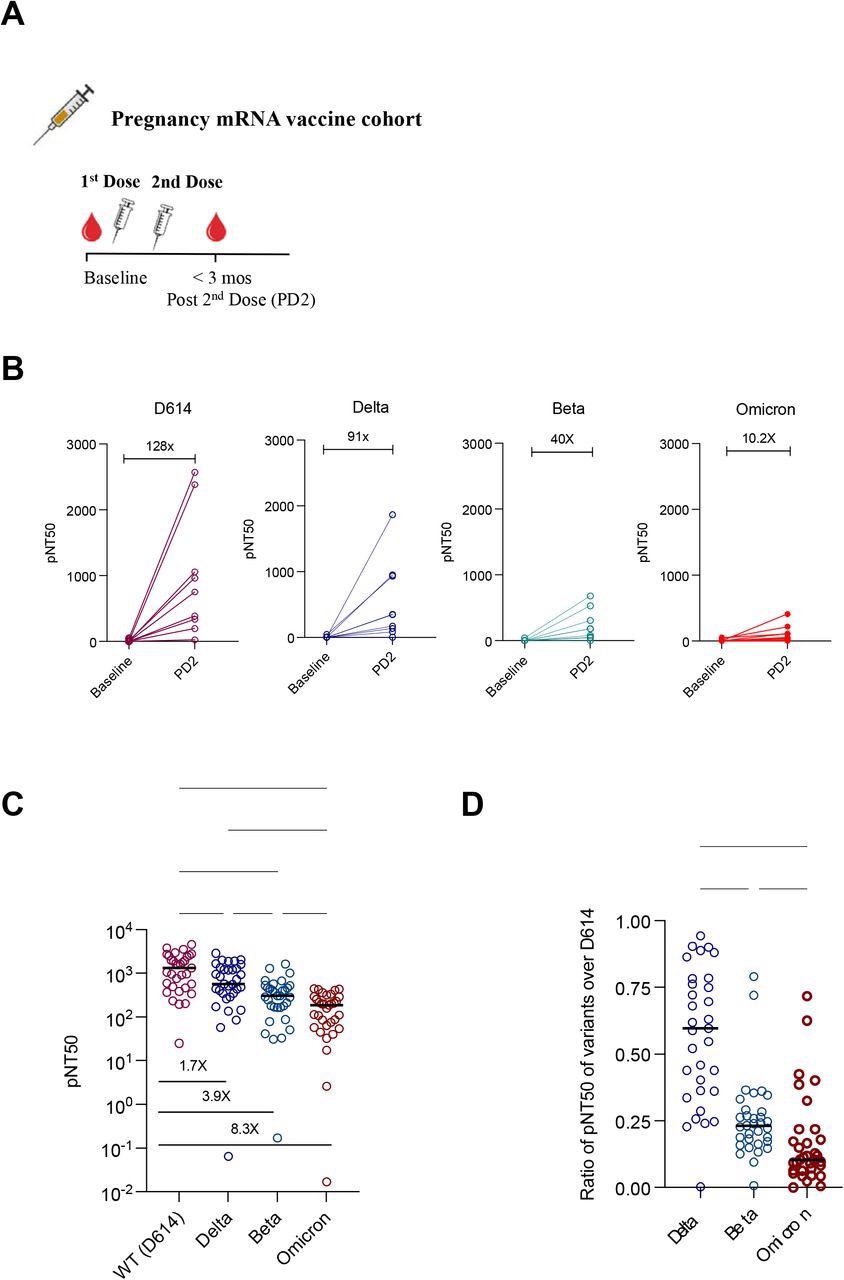
Plasma neutralizing titers against variants of concern in a mRNA vaccinated cohort of pregnant women. (A) Samples collected from a cohort of vaccinated pregnant women at baseline (n=9) and after 2 doses of mRNA vaccine (n=33) were assessed for neutralizing antibody against 4 SARS-CoV-2 pseudovirus strains: D614, Delta (B.1.617.2), Beta (B.1.351) and Omicron (B.1.1.529). (B) Half-maximal SARS-CoV-2 pseudovirus neutralizing titers (pNT50) against D614G, Delta (B.1.617.2), Beta (B.1.351) and Omicron (B.1.1.529) of paired samples at baseline and 2 doses of mRNA vaccine. Fold change of mean titers between the 2 time-points are shown. (C) pNT50 values of samples collected after 2 doses of mRNA against the 4 SARS-CoV-2 pseudoviral variants. Fold reduction of mean pNT50 compared to D614G are shown for each variant. (D) Ratio of pNT50 values of the variants of the concern Delta, Beta and Omicron over D614 pNT50. p values in (C-D) were calculated using mixed-effects analysis with Geisser-Greenhouse correction and Tukey’s multiple comparisons test.
The magnitude of neutralizing titers was 91 times greater against Delta, 40 times greater against Beta, and 10.2 times greater against Omicron. The results suggest vaccines provide a wide range of neutralizing antibody responses among vaccinated individuals who are pregnant.
Antibody response in non-pregnant individuals after vaccination and booster
A total of 137 serum samples were collected from healthcare workers vaccinated with the Pfizer-BioNTech vaccine at Stanford Hospital Center. The team collected samples at various time points: a week after the second dose or 28 days after enrollment as well as 210 days after study enrollment to better understand the durability of neutralizing responses after two vaccine doses.
Neutralizing antibody responses were measured 7 days after the third dose or between 3 to 4 weeks.
The results showed neutralizing antibody responses were highest against D614 and all variants a week after the second and third dose.
As seen in the naturally infected cohorts, antibody titers waned, and neutralizing activity decreased several months after the second dose. In contrast, the booster shot significantly elevated neutralizing antibody titers against all SARS-CoV-2 variants to a level that matched or surpassed antibody levels 7 days after the second dose.
Three to four weeks after the third dose, some individuals showed reduced antibody levels while others maintained antibody levels similar to what researchers observed a week after the booster.
Neutralizing antibody titers were substantially lower against Omicron, suggesting a heterologous antibody response similar to pregnancy and vaccinated individuals, though antibody titers were typically lower in pregnancy.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Sievers BL, et al. (2022). Magnitude and breadth of neutralizing antibody responses elicited by SARS-CoV-2 infection or vaccination. medRxiv. Doi: https://doi.org/10.1101/2021.12.30.21268540, https://www.medrxiv.org/content/10.1101/2021.12.30.21268540v1
- Peer reviewed and published scientific report.
Sievers, Benjamin L., Saborni Chakraborty, Yong Xue, Terri Gelbart, Joseph C. Gonzalez, Arianna G. Cassidy, Yarden Golan, et al. 2022. “Antibodies Elicited by SARS-CoV-2 Infection or MRNA Vaccines Have Reduced Neutralizing Activity against Beta and Omicron Pseudoviruses.” Science Translational Medicine 14 (634). https://doi.org/10.1126/scitranslmed.abn7842. https://www.science.org/doi/10.1126/scitranslmed.abn7842.