The recent emergence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant of concern (VOC) has led to a new wave of coronavirus disease 2019 (COVID-19) cases globally. The increased virulence of the Omicron variant has allowed for this new strain of SARS-CoV-2 to displace the previously dominant Delta variant throughout most countries in the world.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The third COVID-19 wave imposed by the Omicron VOC impacted India in January 2022. The Omicron variant has higher transmissibility and immune escape capabilities that have been implicated in its rapid spread and numerous breakthrough and re-infections.
However, the resultant disease following infections with this variant appears to be less severe compared to that with other VOCs. It is therefore important to ascertain the potential for immune evasion of the Omicron variant among vaccinated individuals and those who have had natural infection.
About the study
A recent study published on the bioRxiv* preprint server analyzed the immunoglobulin G (IgG) and neutralizing antibodies (NAbs) against the SARS-CoV-2 B.1, Alpha, Beta, Delta, and Omicron strains with the sera of individuals infected with the Omicron variant (B.1.1529 and BA.1).
Twenty-eight of the 39 participants selected for the current study were foreign returnees, whereas the remaining 11 were their high-risk contacts confirmed to be infected with the Omicron variant by next-generation sequencing (NGS). Sera samples were collected from all participants at about 10 days after the onset of symptoms. Oro/nasopharyngeal swabs of eight individuals were positive for SARS-CoV-2.
The study participants were categorized based on the vaccines they received. Group 1 included 25 individuals who received the ChAdOx1 nCoV-19 vaccine and experienced a breakthrough infection at an average of 147 after receiving their second dose.
Group 2 consisted of eight individuals who received the Pfizer-BioNTech BNT162b2 messenger ribonucleic acid (mRNA) vaccine and experienced a breakthrough infection at an average of 113 after their second dose. Group 3 included six unvaccinated individuals.
Study findings
The mean age of the participants in Group 1 was 39 years. Among these, 13 were asymptomatic, while twelve reported having mild fever, sore throat, and cough lasting for one to three days. Of these, two cases were of reinfection, with the last SARS-CoV-2 infection documented in July 2020.
The mean age of the participants in Group 2 was 33 years. Among these, only one participant had mild fever and sore throat, while the remaining were asymptomatic.
Meanwhile, the unvaccinated group consisted of only female participants of a mean age of 16 years. Five of the participants in this group were pediatric patients. In this group, four patients were asymptomatic, whereas two pediatric patients were mildly symptomatic.
The results of the current study showed that the geometric mean titers (GMTs) of the S1-receptor-binding domain (RBD) IgG antibodies in the sera of the Group 1 and Group 2 individuals were similar. However, nucleocapsid (N) protein GMTs were 2.5-3.6 times reduced in the sera of Group 2 individuals as compared to those of Group 1.
Group 3 showed varied responses in the GMTs of S1-RBD IgG antibodies.
Group 2 individuals also exhibited significant reductions in their GMTs against the Delta and Omicron variants at 1.52- and 7.41-fold, respectively. Notably, the participants in this group did not have a significant reduction in their GMTs against the Alpha and Beta variants.
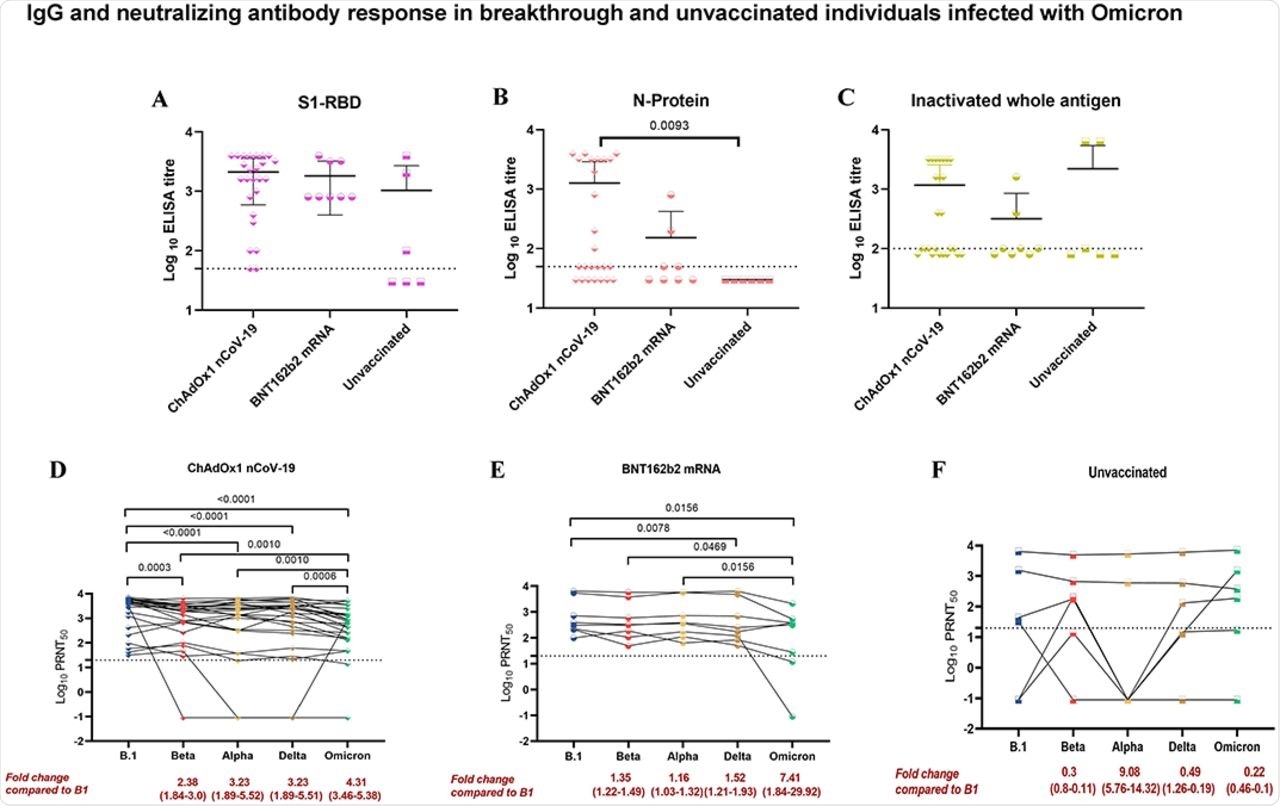
IgG antibody response in breakthrough and unvaccinated individuals was assessed using ELISA.
Implications
Taken together, the researchers observed a three-fold reduction in NAb titers in BNT162b-vaccinated breakthrough infections when compared to ChAdOx1 nCoV-19 breakthrough infections. Furthermore, the researchers found that individuals infected with the SARS-CoV-2 Omicron variant harbor a significant immune response against most other SARS-CoV-2 VOCs, including the Delta variant.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Yadav, P., Sapkal, G., Sahay, R., et al. (2022). Substantial immune response in Omicron infected breakthrough and unvaccinated individuals against SARS-CoV-2 variants of concerns. bioRxiv. doi:10.1101/2022.01.24.477043. https://www.biorxiv.org/content/10.1101/2022.01.24.477043v1.
- Peer reviewed and published scientific report.
Yadav, Pragya D., Gajanan N. Sapkal, Rima R. Sahay, Varsha A. Potdar, Gururaj R. Deshpande, Deepak Y. Patil, Dimpal A. Nyayanit, et al. 2022. “Substantial Immune Response in Omicron Infected Breakthrough and Unvaccinated Individuals against SARS-CoV-2 Variants of Concern.” Journal of Infection, February. https://doi.org/10.1016/j.jinf.2022.02.005. https://www.journalofinfection.com/article/S0163-4453(22)00070-6/fulltext.