The coronavirus disease 2019 (COVID-19) pandemic was caused by the rapid spread of coronavirus disease-2 (SARS-CoV-2). To date, this pandemic has claimed more than 5.77 million lives worldwide.
Vaccine availability has increased, but limited longevity of vaccine-induced immunity, declining vaccine uptake among some segments of the population, and increasingly contagious and/or drug-resistant variants (VOCs) such as Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Omicron (B1.1.529) have accelerated global infection waves.
Molnupiravir, a direct-acting antiviral administrated orally, promises to aid in disease management and enhance SARS-CoV-2 containment. Molnupiravir's potency against Omicron has not been thoroughly explored, and clinical trial results raise questions about its effectiveness against VOC Delta.
A study published on the bioRxiv* preprint server evaluates molnupiravir against a panel of relevant VOCs using three efficacy models: primary human airway epithelium organoids, a ferret model of upper respiratory disease, and a Roborovski dwarf hamster model for severe COVID-19.
Study: SARS-CoV-2 variant of concern type and biological sex affect efficacy of molnupiravir in dwarf hamster model of severe COVID-19. Image Credit: Naeblys/Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The summer and autumn of 2021 witnessed the growth of VOC delta due to its replication to high titers, prolonged shedding, and ability to cause breakthrough infections in vaccinees.
As of November 2021, VOC omicron had overtaken Delta as the dominant strain circulating in most regions, largely due to its significantly reduced susceptibility to antibodies directed against earlier lineages and increased infectiousness. VOC omicron may have milder clinical symptoms but its high daily infection rates have led to high hospitalization rates, which makes the need for therapeutics to improve disease management urgent.
Molnupiravir, a SARS-CoV-2 inhibitor, was the first approved drug that supported oral administration to treat SARS-CoV-2 infection. Early reports on clinical trials associated with molnupiravir treatment revealed a significant reduction in hospitalizations in the treated group. In contrast, the later trial phase showed lower efficacy of molnupiravir against the Delta variant. However, ex vivo studies have demonstrated that molnupiravir parent metabolite N4 -hydroxycytidine (NHC) could efficiently inhibit the Delta VOC.
Some of the newly developed preclinical models to assess the efficacy of anti-SARS-CoV-2 drug candidates are human airway epithelium organoids, ferrets, mice, and Syrian golden hamsters as preclinical models. Ferrets represent the clinical manifestation of SARS-CoV-2 infection in younger patients, characterized by a high viral load in the upper respiratory tract and strong viral shedding. Syrian golden hamsters infected by SARS-CoV-2 represent mild-to-asymptomatic infection. Although transgenic K18-hACE2 mice represent severe COVID-19 infection, it develops lethal viral encephalitis, which is not similar to human symptoms. Hence, there is a need for an efficacy model that can help determine the effectiveness of molnupiravir in mitigating lung damage caused by SARS-CoV-2 VOCs.
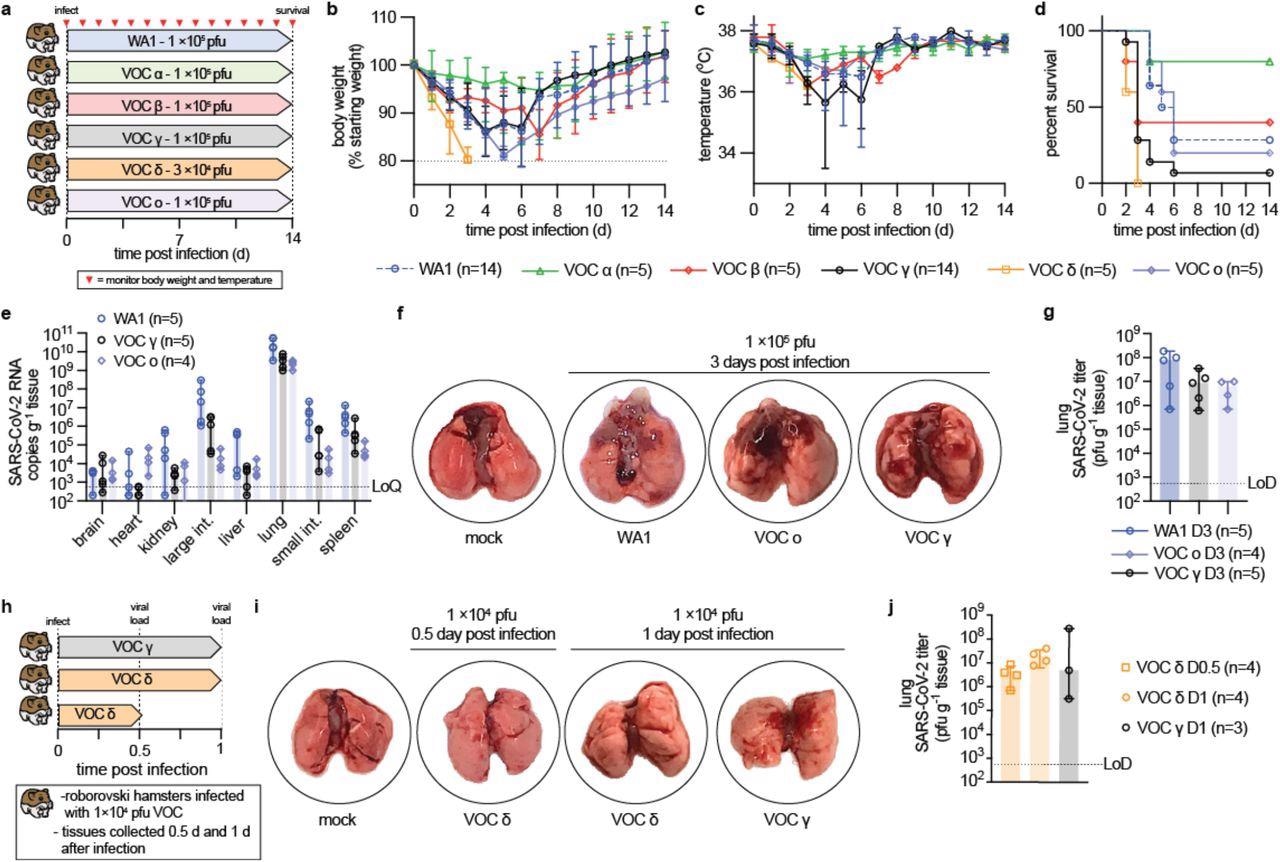
VOC pathogenesis in Roborovski dwarf hamsters. a, Schematic of the dwarf hamster pathogenesis study. Animals were monitored for up to 14 days after intranasal infection with 1 × 105 pfu each (VOC delta 3 × 104 pfu per animal). Clinical signs were assessed once daily (red triangles). b-c, Bodyweight (b) and temperature (c) of infected dwarf hamsters. d, Survival curves of infected dwarf hamsters from (a). e, Viral RNA copies in select organs extracted from infected hamsters 3 days after infection. f, Images of lungs from hamsters mock-infected or inoculated with 1 × 105 pfu WA1, VOC omicron, or VOC gamma 3 days after infection. g, Infectious titers from the lungs of hamsters shown in (f). h, Schematic of the dwarf hamster pathogenesis study utilizing 1 × 104 pfu VOC delta and gamma. i, Images of lungs extracted from hamsters mock-infected or inoculated with 1 × 104 pfu VOC omicron or VOC gamma 0.5 (delta) and 1 day (delta and gamma) after infection. j, Infectious titers from the lungs of hamsters shown in (i). Symbols represent independent biological repeats (e, g, j), lines intersect group medians (b-c), columns show group medians (e, g, j), and error bars represent 95% 0 confidence intervals. LoD, limit of detection; LoQ, limit of quantitation.
The Study
The scientists selected Roborovski dwarf hamsters as a candidate model to evaluate the efficacy of molnupiravir against SARS-CoV-2 VOCs. They chose these dwarf hamsters because they develop acute diffuse alveolar pneumonia when infected with SARS-CoV-2 original strain. The study aimed to determine the effect of molnupiravir against the Alpha, Beta, Gamma, Delta, and Omicron strains.
Key Findings
The current study exhibited the efficacy of molnupiravir against SARS-CoV-2 VOCs in human airway organoids, the ferret transmission model, and the Roborovski dwarf hamster model of acute pneumonia.
Researchers observed antiviral potency of the molnupiravir parent NHC against all VOCs, including the Delta and Omicron variants.
Previous studies claimed that a high genetic barrier prevents viral escape, as well as a wide range of antiviral activity of molnupiravir, promotes high efficacy against genetically similar viruses, i.e., VOCs.
The authors further reported that treatment with molnupiravir considerably decreased VOC shedding from the upper respiratory tract of ferrets. This implies that molnupiravir treatment reduced the period of infectiousness of the host.
This study reported no VOC-specific differences regarding the drug's efficacy were observed. Therefore, the varied clinical performance of molnupiravir during the early and late phases of clinical trials could be due to the lower effectiveness of the drug against the Delta variant.
The researchers stated that individual VOCs could indirectly manipulate molnupiravir pharmacokinetic properties differentially by spreading to organs, other than lungs, with specific kinetics.
The study demonstrated that in dwarf hamsters, low viral RNA burden was found in the liver, which is the primary site of drug metabolism.
Interestingly, the researchers observed that the biological sex of the animals played a vital role in determining the therapeutic benefit of molnupiravir use against the Omicron, i.e., males exhibited better reaction compared to females. However, such differences in efficacy were not observed for other SARS-CoV-2 variants.
Conclusion
The high efficacy of molnupiravir in controlled conditions using animal models compared to human trials remains elusive. Due to the absence of controlled clinical data associated with molnupiravir efficacy against Omicron, the relevance of dwarf hamster-derived results to human therapy is not clear.
The authors recommend continuous assessment of the therapeutic benefit of approved antivirals against newly emerged VOCs.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Lieber, M.C. et al. (2022) SARS-CoV-2 variant of concern type and biological sex affect efficacy of molnupiravir in dwarf hamster model of severe COVID-19. bioRxiv 2022.02.04.479171; doi: https://doi.org/10.1101/2022.02.04.479171, https://www.biorxiv.org/content/10.1101/2022.02.04.479171v1
- Peer reviewed and published scientific report.
Lieber, Carolin M., Robert M. Cox, Julien Sourimant, Josef D. Wolf, Kate Juergens, Quynh Phung, Manohar T. Saindane, et al. 2022. “SARS-CoV-2 VOC Type and Biological Sex Affect Molnupiravir Efficacy in Severe COVID-19 Dwarf Hamster Model.” Nature Communications 13. https://doi.org/10.1038/s41467-022-32045-1. https://www.nature.com/articles/s41467-022-32045-1.