I was born and raised in Ethiopia. I obtained my M.D. and MSc in Medical Microbiology from Addis Ababa University, Ethiopia, and my MSc in Vaccinology & Pharmaceutical Clinical Development from the University of Siena, Italy. I have more than 10 years of clinical research experience.
I have been a full-time employee of Novartis, GlaxoSmithKline, and the Bill & Melinda Gates Medical Research Institute. Some of my key responsibilities in these three roles include contributing to vaccine development clinical strategy and critical contributions to clinical documents, including clinical trial protocols, reports and publications, and integrated clinical documents for regulatory submissions. It has also involved identifying study sites for clinical trials, training them on the study protocol and other related documents, and serving as a medical monitor.
At Novartis and GSK, I worked on Influenza, Meningococcal, and Zoster vaccine clinical development programs. I contributed to the design and execution of clinical studies and critical interactions with the U.S. Food & Drug Administration (FDA), European Medicines Agency (EMA), Health Canada, and other regulatory agencies. I have also published sixteen manuscripts in peer-reviewed journals, and two more are in preparation.
I have been working on tuberculosis vaccine clinical development in my current role at Bill & Melinda Gates Medical Research Institute (Gates MRI) since 2020.
I know the burden of Tuberculosis first-hand from my previous practice as a physician and researcher in Ethiopia. It really was a great opportunity for me to join Gates MRI and use the many years of experience I have gained by working for pharmaceutical companies, to help develop a vaccine with the potential for a very significant impact to the community I came from, and beyond.
What makes tuberculosis (TB) the world's most infectious killer? - Melvin Sanicas
TB is currently in the top 13 leading causes of death worldwide with 1.5 million people dying from this in 2020. What makes TB so infectious?
Tuberculosis (TB) is caused by the bacterial species Mycobacterium tuberculosis (Mtb) that most often affects the lungs. Infection with Mtb typically occurs following exposure to Mtb-containing aerosol droplets transmitted from an individual with active TB disease. Infection most often results in the establishment of a “latent” infection status, which can convert to active TB disease at any time during the latently infected individual’s lifetime.
When a person develops active TB disease, the symptoms (such as cough, fever, night sweats, or weight loss) may be mild for many months. This can lead to delays in seeking treatment, thereby prolonging the period of transmissibility.
TB bacteria are spread through the air from one person to another. The Mtb bacteria are put into the air when a person with active TB disease of the lungs or throat coughs, speaks, or sings. People nearby may breathe in these bacteria and become infected.
"It really was a great opportunity for me to join Gates MRI and use the many years of experience I have gained by working for pharmaceutical companies, to help develop a vaccine with the potential for a very significant impact to the community I came from, and beyond."
Despite this disease affecting so many people worldwide, particularly within developing countries, it is curable and preventable. What treatment options are currently available for TB and what more needs to be done to ensure global access to these treatments?
Currently, bacille Calmette-Guérin, known as BCG, is the only licensed TB vaccine. However, it has limited impact on the prevention of Mtb infection and prevention of TB disease in adults. Treatment of TB disease is curative but lengthy, and compliance is often incomplete. The global prevalence of drug-resistant TB is substantial.
Improved prevention tools and effective vaccines to prevent the development of TB disease are urgently needed to accelerate progress toward ending the TB epidemic. However, since the introduction of the BCG vaccine in 1921, very few novel vaccine candidates have advanced into clinical efficacy trials.
There has been years of global progress in tackling the TB epidemic, yet according to the World Health Organization’s 2021 Global TB Report, deaths for the first time in a decade have increased. What impact has the ongoing COVID-19 pandemic had on TB?
The COVID-19 pandemic has reversed years of global progress in tackling tuberculosis. There was a substantial reduction in TB case detection and reporting between 2019 and 2020, which is likely a result of reduced health system capacity, less willingness to seek care during lockdowns, risks of going to a health care facility during a pandemic, and stigma associated with similarities in the symptoms of TB and COVID-19.
The most immediate consequence of this was the increase in the number of people who died from TB in 2020, at all levels: global, regional, and country. Around the world in 2020, there were an estimated 1.3 million TB-caused deaths among HIV-negative people, up from 1.2 million in 2019, and 214,000 deaths among HIV-positive people.
"The COVID-19 pandemic has reversed years of global progress in tackling tuberculosis."
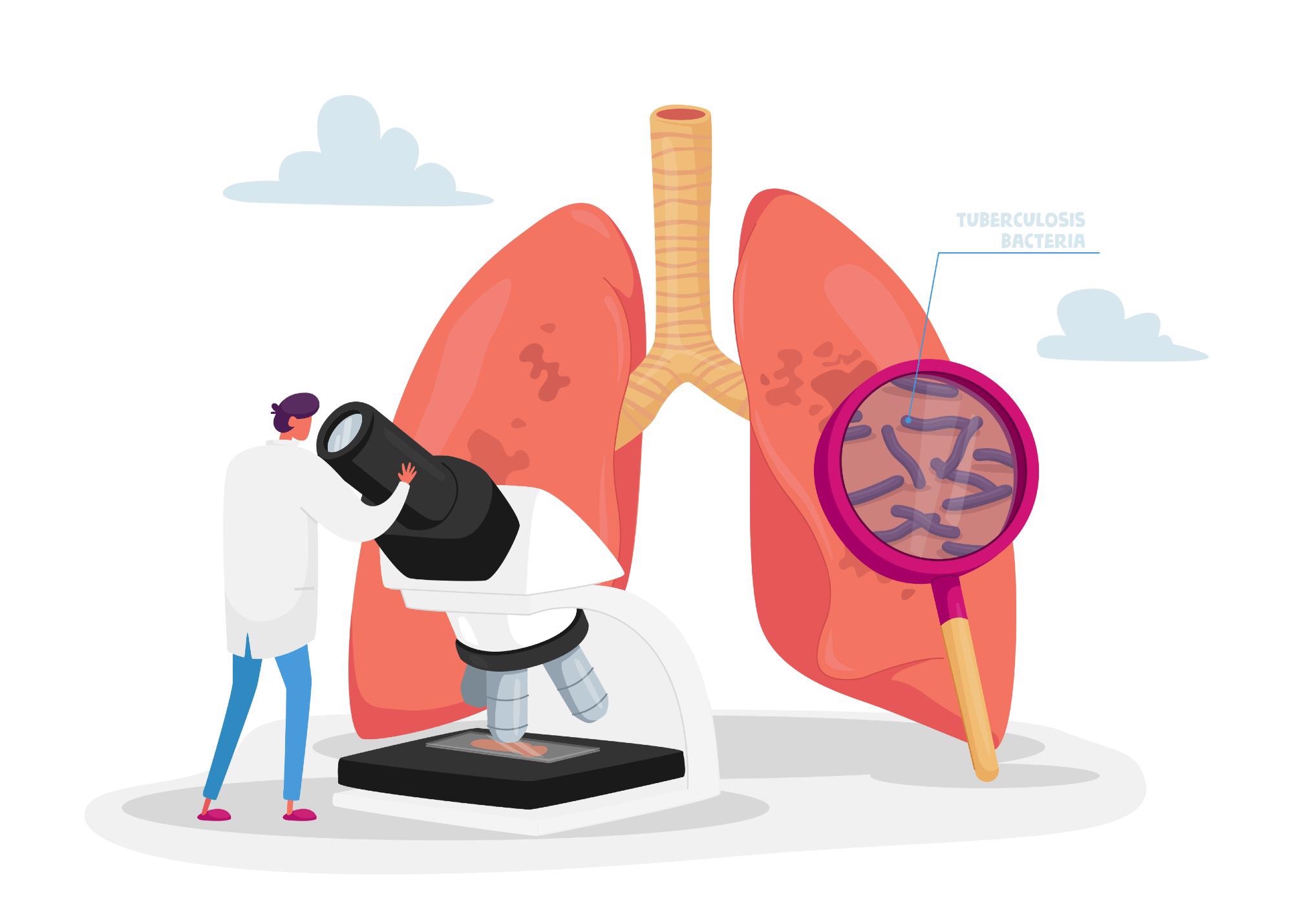
Image Credit: lemono/Shutterstock.com
Currently, only one tuberculosis vaccine has been developed which highlights the urgent need for continued innovation within this space. What role does the Gates Medical Research Institute (Gates MRI) play within the TB epidemic?
At Gates MRI, we strive to address substantial global health concerns, for which investment incentives are limited. Tuberculosis is an infectious disease in great need of attention, it has a particularly devastating impact on people in many low- and middle-income countries. Developing a new vaccine against TB is a global health priority to accelerate progress toward ending the TB epidemic, a key element of the United Nations Sustainable Development Goals.
The investigational M72/AS01E vaccine was developed by GSK, supported by grant funding from the Bill & Melinda Gates Foundation (BMGF) and by Aeras, for the prevention of TB disease in Mycobacterium tuberculosis (Mtb)-infected adolescents and adults. M72/AS01E is not intended to replace BCG, the live-attenuated TB vaccine given at birth. The Gates MRI has primary responsibility for the investigational vaccine’s ongoing development, including a phase 3 efficacy trial.
Gates MRI is also currently conducting a Phase 2 trial in South Africa with M72/AS01E, referred to as the MESA-TB trial, to evaluate the safety and immunogenicity of the vaccine candidate in people living with HIV (PLHIV). This trial is important given that PLHIV represents a substantial portion of the TB disease burden.
You are currently conducting an epidemiologic study for a candidate vaccine for TB. Can you describe how you are carrying out this study? What do you hope to see?
In anticipation of a phase 3 efficacy trial of the investigational M72/AS01E vaccine, the Gates MRI is conducting an epidemiology study to document the prevalence of latent Mtb infection and the incidence of TB disease in populations with a high TB disease burden.
Since the national incidence rate of active TB is relatively low in most high-burden countries, it is critical that the trial is conducted in a general population living in communities with as high a TB incidence rate as possible for the efficacy trial to be feasible with regards to sample size, trial duration, and cost.
Site selection for the epidemiology study was focused on identifying clinical trial sites in communities with very high TB incidence rates in adolescents and young adults to establish capacity for the Phase 3 efficacy trial of the investigational M72/AS01E TB vaccine. Thirty countries and over 200 sites were included in the country and site feasibility assessment and 50 sites in 14 countries were selected.
The epidemiology study will establish operational capacity and feasibility for each site for a phase 3 trial with regard to study participant recruitment, follow-up, retention, and biological sample processing procedures.
Locations were selected based on key factors, including high demonstrated incidence, geographic representation, start-up complexity, and recruitment capacity.
Currently projected site activations by country include: South Africa, Peru, Kenya, Gambia, Zambia, Brazil, Uganda, Vietnam, Philippines, Mozambique, India, DRC, Bangladesh, and Indonesia.
Gates MRI is investing in this study to not only prepare clinical sites for potential inclusion in an M72/AS01E clinical efficacy trial, but also to identify clinical study sites that can be used for the assessment of other future potentially effective TB vaccines.
"Developing a new vaccine against TB is a global health priority to accelerate progress toward ending the TB epidemic, a key element of the United Nations Sustainable Development Goals."
Image Credit: Novikov Aleksey/Shutterstock.com
Are you hopeful that with promising results from your study, it will help to support the vaccines phase 3 trial? What more needs to be done before this vaccine can be rolled out globally?
The primary purpose of the epidemiology study is to assess the interferon-gamma release assay (IGRA) positivity at each site in order to identify sites with populations at high risk of Mycobacterium tuberculosis (Mtb) infection. IGRA positivity is an indicator of Mtb infection. The study will also serve to build capacity to conduct the planned vaccine efficacy study.
Study site selection is an important factor in maximizing the probability of success for the planned TB vaccine efficacy study. Accurate, relevant, site-specific epidemiology data will enable a robust trial size for the Phase 3 efficacy trial, and this selection process takes time.
The ability to start these studies depends on observing a predefined number of TB cases, which in turn depends upon the speed of enrollment and incidence rate. We have made significant progress and are working with key stakeholders and institutions globally to prepare sites for the start of the phase 3 trial.
What do you hope the future of TB to look like worldwide? Are you hopeful that one day we will see a world without tuberculosis?
TB can be preventable and curable with the right investments and resources, and although there is progress in the development of new TB diagnostics, drugs, and vaccines, this is constrained by the overall level of R&D investment.
With continued investment, both monetary and scientific, I am confident that a world without TB is attainable.
The Gates MRI helps to address global health concerns by collaborating with partners and organizations. How important is collaboration to Gates MRI? What advantages does global collaboration have for innovation within healthcare?
The institute works in close collaboration with R&D partners and organizations, coordinating and driving the full spectrum of biopharmaceutical development activities, including pre-clinical development, full clinical development (from phase 1 through to and including phase 3), and global regulatory interactions.
Global collaboration brings new perspectives to inform innovation, provides the resources to make sustainable change, and allows us to scale our portfolio of potential drug and vaccine candidates to a global level.

Image Credit: STILLFX/Shutterstock.com
Are there any other upcoming projects/studies that you are involved in at Gates MRI?
In the Gates MRI TB vaccine program, the next big study is the phase 3 efficacy trial of the investigational M72/AS01E vaccine.
Where can readers find more information?
About Dr. Alemnew Dagnew
Alemnew Dagnew, M.D., MSc., is a Clinical Development Leader at the Bill & Melinda Gates Medical Research Institute. He joined Gates MRI in 2020 after more than ten years with Novartis and GSK Vaccines. He started his research career in Ethiopia by working on TB immunology and TB vaccine clinical trials.
Dr. Dagnew received his M.D. from Addis Ababa University, Ethiopia, MSc in Medical Microbiology from Addis Ababa University, Ethiopia, and MSc in Vaccinology & Pharmaceutical Clinical Development from the University of Siena, Italy.