The sudden and rapid outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused the coronavirus disease 2019 (COVID-19) pandemic, which has claimed more than 6.3 million lives worldwide as of June 7, 2022. Due to the high rate of genomic mutation, several SARS-CoV-2 variants have emerged, which have been categorized as variants of concern (VOC) and variants of interest (VOI).
Researchers have reported that SARS-CoV-2 affects many organs including the lungs, stomach, and heart of infected patients. In a recent Viruses review article, scientists discuss the gastrointestinal (GI) complications associated with COVID-19.
Study: Gastrointestinal Involvement in SARS-CoV-2 Infection. Image Credit: Kateryna Kon / Shutterstock.com
SARS-CoV-2 infection and GI complications
Several studies have reported that SARS-CoV-2 primarily targets the lung cells, which causes significant respiratory complications. Interestingly, many studies have also reported the presence of SARS-CoV-2 ribonucleic acid (RNA) in the stool samples of infected patients, thus confirming the shedding of SARS-CoV-2 in feces.
Some of the common GI complications associated with COVID-19 include vomiting, anorexia, nausea, and diarrhea. SARS-CoV-2 infection with GI symptoms might result in acute infection with a poor prognosis.
GI imaging in COVID-19 patients has provided evidence of a thickening of the bowel wall, mesenteric thickening, fluid-filled large bowels, hyperemia, pneumatosis, and ischemia on rare occasions. Previous studies have also reported that diarrhea caused by SARS-CoV-2 infection might be due to malfunctioning of intestinal ion transporters that cause inflammation and various GI complications.
Importantly, COVID-19 patients suffering from GI symptoms are often more likely to develop severe respiratory distress. Scientists have speculated that inflammatory cytokines could be the possible connection in the SARS-CoV-2 pathogenesis between the respiratory and digestive systems.
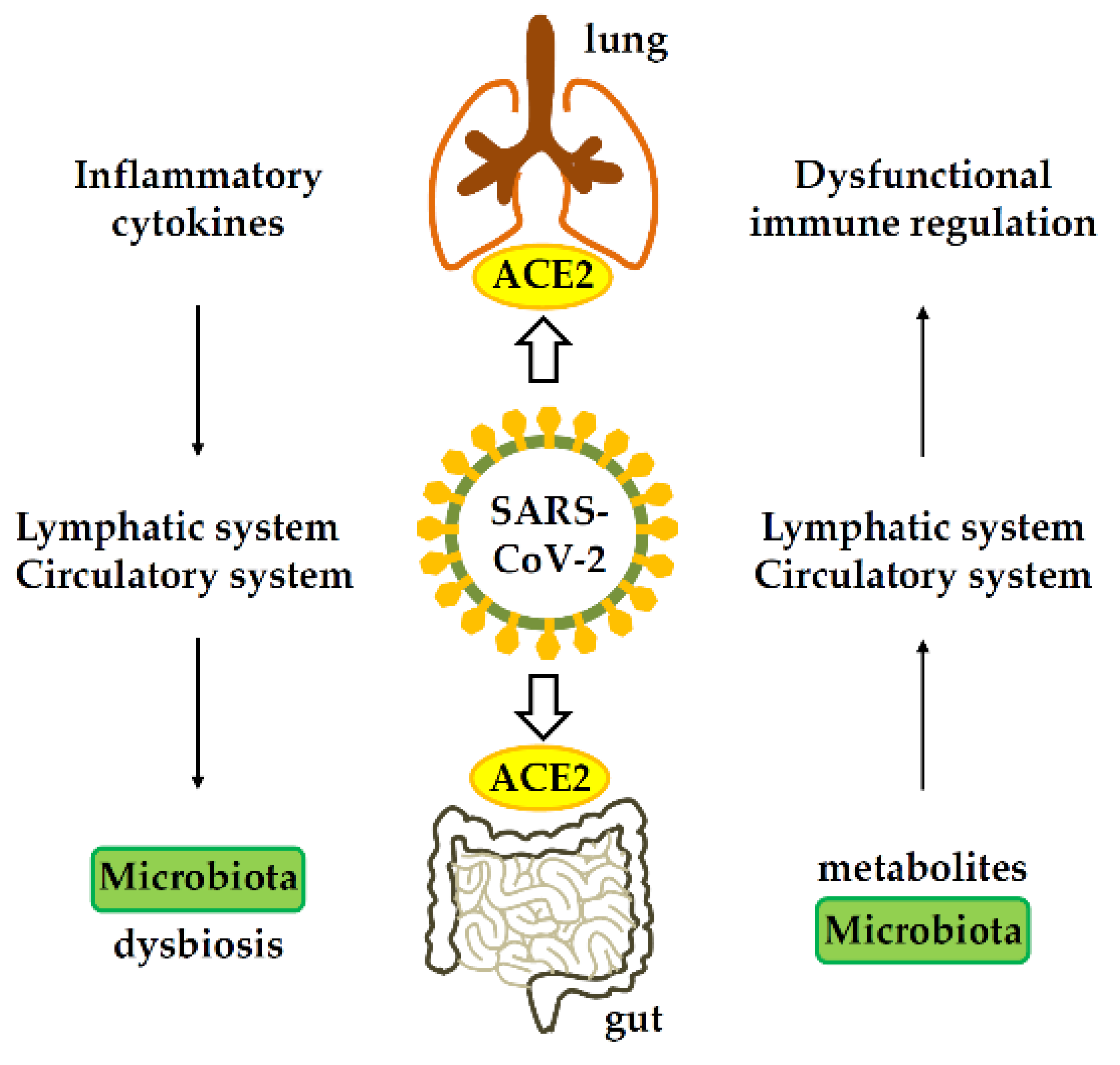 Gastrointestinal-lung axis in COVID-19. ACE2, angiotensin-converting enzyme 2.
Gastrointestinal-lung axis in COVID-19. ACE2, angiotensin-converting enzyme 2.
Moreover, patients with acute COVID-19 experience more abdominal pain than patients with mild symptoms. However, not much difference has been reported with regards to loss of appetite, vomiting, diarrhea, and nausea symptoms reported in both groups of patients.
Patients suffering from thromboembolic events and GI system disorders such as mesenteric ischemia are at a high risk of mortality. Additionally, the level of transaminase in COVID-19 patients is typically very high, which results in intestinal ischemia and elevates the risk of intestinal obstruction.
Previous studies have also indicated that SARS-CoV-2 virions can enter the GI tract through the esophagus. Furthermore, detection of SARS-CoV-2 in the stool of the infected patients implies that the virus has been transmitted through the fecal-oral route. Taken together, endoscopic sampling of a COVID-19 patient’s GI tract has revealed the presence of SARS-CoV-2 RNA in the stomach, esophagus, rectum, and duodenum. The SARS-CoV-2 nucleocapsid (N) protein 2 has also been detected in the cytoplasm of rectal glandular epithelial cells and duodenal cells.
SARS-CoV-2 and the gut microbiome
The presence of viruses in the GI tract influences the hosts health, as the virus interacts with the mucus layers, lamina propria immune cells, and epithelial cells. Furthermore, alterations in the gut virome can have a significant impact on the immunophenotype.
The gut microbiome is rich in beneficial bacteria that are responsible for maintaining intestinal homeostasis, suppressing excessive mucosal inflammation, and facilitating the development of immune responses at mucosal surfaces. Taken together, the gut microbiota consists of approximately 100 trillion microorganisms and thousands of bacterial species.
Adaptive and innate immune cells are triggered by the disruption of gut barrier integrity. Additionally, the release of pro-inflammatory cytokines into circulation can lead to systemic inflammation. Thus, the entry of inflammatory cells like neutrophils and lymphocytes into the intestinal mucosa can cause severe disruption of the gut microbiota.
A change in the composition of the gut microbiome, i.e., an increase in Campylobacter, Parabacteria, Bacteroides, Bifidobacterium, Clostridium, Ruminococci, Rotella, Corynebacterium Pseudomonas, Enterococcus, and Aspergillus, and a considerable reduction in Eubacterium, Faecalibacterium, Lachnospira, and Firmicutes, influences COVID-19 outcomes. One previous study has indicated that alterations in the composition and function of the gut microbiome affect the respiratory tract via the common mucosal immune system. Respiratory dysbiosis also influences the digestive tract through immune regulation.
SARS-CoV-2 elicits early neutralizing antibody responses including a peripheral expansion of immunoglobulin A (IgA) plasmablasts with mucosal homing potential, systemic IgA, and systemic IgG. One previous study has reported that the gut-lung axis plays an important role in controlling COVID-19.
Another study revealed that cytokines could enter the lungs through the bloodstream when the intestine is inflamed. This condition significantly affects pulmonary immune responses and inflammation.
An increase in circulating pro-inflammatory cytokines could also impact the composition of the gut microbiome which, in turn, could enhance intestinal permeability. This may cause translocation of pathogens and toxins and, as a result, enhance disease severity and lead to multiple organ failures.
An altered gut microbiome and epithelial inflammation could also enhance the expression of the angiotensin-converting enzyme 2 (ACE2) receptor in the gut, which is primarily used by SARS-CoV-2 to gain entry into cells.
Taken together, the exact underlying mechanisms associated with common GI symptoms and COVID-19 remain largely unknown.
Conclusions
Some COVID-19 patients suffer from GI symptoms; however, these off-target symptoms in SARS-CoV-2 infected patients are often neglected. In the future, GI symptoms and changes in the gut microbiota of COVID-19 patients must be studied, as targeting these tissues might be effective in controlling the infection.
Journal reference:
- Chen, H. T., Hsu, M., Lee, M., et al. (2022) Gastrointestinal Involvement in SARS-CoV-2 Infection. Viruses 14(6). doi:10.3390/v14061188.