Exosomes are endosome-origin extracellular vesicles that mediate events involved in inter-cellular events by transporting functional molecules associated with tissue homeostasis. Exosomes have been used in cancer diagnosis and therapy; however, whether defensosomes (an exosome subset) can widen the diagnostic and therapeutic landscape of viral diseases needs further investigation.
The present study's authors previously showed that defensosomes mediate immune protection against bacterial toxins in an autophagy protein (ATG16L1)-dependent manner via incorporating proteinaceous receptors on the surface. They also showed that activation of toll-like receptor 9 (TLR9) induces exosomes in response to bacterial deoxyribonucleic acid (DNA) or CpG-A.
 Study: ACE2-containing defensosomes serve as decoys to inhibit SARS-CoV-2 infection. Image Credit: Meletios Verras / Shutterstock
Study: ACE2-containing defensosomes serve as decoys to inhibit SARS-CoV-2 infection. Image Credit: Meletios Verras / Shutterstock
About the study
In the present study, researchers explored defensosomes as a potential biomarker and therapeutic agent against SARS-CoV-2.
The study comprised 80 adults with RT-PCR (reverse transcription-polymerase chain reaction)-confirmed acute coronavirus disease 2019 (COVID-19) admitted to the intensive care unit (ICU) due to respiratory failure with mechanical ventilation requirements. Bronchoalveolar lavage fluid (BALF) samples were obtained from the study participants to investigate the generation and presence of ACE2+ exosomes in the lower airways by flow cytometry (FC) analysis.
Plaque assays determined SARS-CoV-2 titers, and in vitro neutralization assays were performed using human airway epithelial cultures (HAECs) to assess humoral antibody responses against anti-SARS-CoV-2 nucleocapsid (N) protein. In addition, RNA sequencing (RNA-seq) was performed to analyze the dACE2 (dACE2) protein, and the mean fluorescence intensity (MFI) was evaluated to assess variations in surface ACE2-containing exosome proportions and ACE2 expression by the exosomes.
Further, the team investigated whether ACE2+ exosome proportions correlate with clinical parameters such as sex, age, COVID-19 severity, and comorbidities such as hypertension and diabetes. They also assessed the possible association between ACE2+ exosome proportions and duration of ICU admission, hospitalization, and ventilator use using linear and negative binomial modeling.
The team assessed the association between ACE2+ exosome production and interferon (IFN) responses, and differential gene analyses were performed for individuals with "high" ACE2 levels on their exosomes or "high" ACE2+ exosome producers. Further, cell culture experiments were performed using A549 cells and Vero E6 cells. Finally, western blot analysis and FC analysis were performed using the Abcam 15348 and AF933 antibodies, respectively, for full-length ACE2 detection.
The possible protection of SARS-CoV-2 USA WA1/2020-infected Vero E6 cells by donor-provided and induced exosomes was assessed, and immunofluorescence microscopy analysis was performed. Further, electron microscopy (EM), cryo-EM, and tomography analysis were performed to visualize the SARS-CoV-2 virion and ACE2+ defensosomes interactions.
Results
Most (76%) of the participants were men, and non-Whites (54%), with an average age of 62. Diabetes and hypertension were reported in 39% and 53% of participants, respectively, and did not significantly impact ACE2+ exosome proportions. The average duration of ICU admission was 48 days. Exosomes containing a higher expression of ACE2 receptors in BALF from critically ill COVID-19 patients were associated with a lower duration of hospitalization, ICU admissions, and ventilator use.
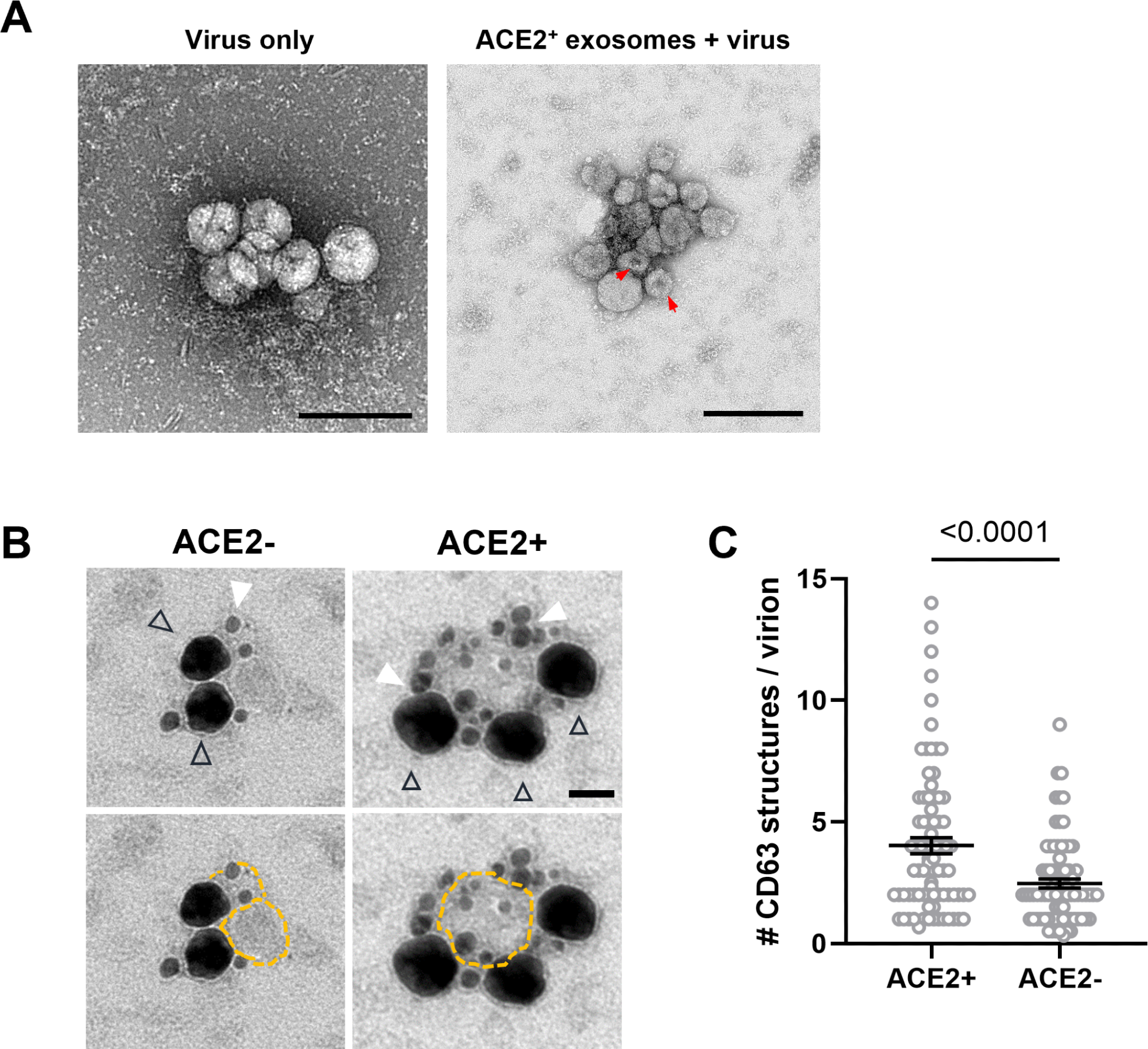 SARS-CoV-2 virions associate with ACE2+ exosomes. (A) TEM of negative stained exosomes and virus alone. Arrowheads: putative exosomes. Scale bars: 200 nm. Red arrows mark the characteristic collapsing of exosomes during TEM imaging. (B) Double immunogold labeling TEM images of SARs-CoV-2 virions mixed with ACE2+ exosomes (right panel, scale bar: 50 nm) or ACE2− exosomes (left panel). Virions are labeled using an antibody for SARS-CoV-2 N, followed by nanogold-conjugated Fab (black triangle outline), which fills the entirety of the virion giving it the appearance of the larger dark mass. Exosomes are labeled using an antibody against CD63, followed by 18-nm colloidal gold-conjugated IgG (filled white triangles). Bottom panels are the same images as the top panels, with exosome membranes marked with dotted yellow outlines denotes to enhance their visibility. (C) Quantification of CD63 structures per virion present in a cluster containing both markers. ACE2+ (n = 68 clusters), ACE2− (n = 57). Error bars represent mean ± SEM from at least two independent experiments. Unpaired t test with Welch's correction. * P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001; **** P ≤ 0.0001. ns, not significant; SARS‑CoV‑2, Severe Acute Respiratory Syndrome Coronavirus 2; TEM, transmission electron microscopy.
SARS-CoV-2 virions associate with ACE2+ exosomes. (A) TEM of negative stained exosomes and virus alone. Arrowheads: putative exosomes. Scale bars: 200 nm. Red arrows mark the characteristic collapsing of exosomes during TEM imaging. (B) Double immunogold labeling TEM images of SARs-CoV-2 virions mixed with ACE2+ exosomes (right panel, scale bar: 50 nm) or ACE2− exosomes (left panel). Virions are labeled using an antibody for SARS-CoV-2 N, followed by nanogold-conjugated Fab (black triangle outline), which fills the entirety of the virion giving it the appearance of the larger dark mass. Exosomes are labeled using an antibody against CD63, followed by 18-nm colloidal gold-conjugated IgG (filled white triangles). Bottom panels are the same images as the top panels, with exosome membranes marked with dotted yellow outlines denotes to enhance their visibility. (C) Quantification of CD63 structures per virion present in a cluster containing both markers. ACE2+ (n = 68 clusters), ACE2− (n = 57). Error bars represent mean ± SEM from at least two independent experiments. Unpaired t test with Welch's correction. * P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001; **** P ≤ 0.0001. ns, not significant; SARS‑CoV‑2, Severe Acute Respiratory Syndrome Coronavirus 2; TEM, transmission electron microscopy.
ACE2+ exosomes were induced by SARS-CoV-2 infection and SARS-CoV-2 sensor activation in cell culture, which required the ATG16L1 protein, defining them as defensosomes. ACE2+ defensosomes were directly bound to SARS-CoV-2, preventing viral entry. Ten patients had ≥2-fold higher exosome levels in their BALF than other participants, indicative of variations between persons concerning the quantity of ACE2 on every exosome and the number of ACE2+ exosomes produced by every person.
SARS-CoV-2 infection of A549ACE2+ cells induced robust ACE2+ exosome production, which could be impaired by the knockdown of ATG16L1. Damage during airway infections led to oxidized mitochondrial DNA (ox-mtDNA) production and therefore enhanced exosome production. CpG-B lacked a phosphodiester backbone, causing poor interferon (IFN) induction and exosome induction failure.
IFN-α, IFN-λ2, and ectopic TLR7 production (upon R848 stimulation) induced exosomes, whereas IFN-β failed to induce exosomes from triple knockout (TKO) A549 cells that lacked essential receptors for type I, III, and III IFN recognition. TKO A549 cells produced exosomes in response to CpG-A. In contrast, the MDA-5 (melanoma differentiation-associated protein 5) and RIG-I (retinoic acid-inducible gene I) cytosolic ribonucleic acid (RNA) sensors did not induce exosomes.
Full-length ACE2 was detected readily using AF933 and Abcam 15348 antibodies, indicating that full-length ACE2 is incorporated into BALF-derived exosomes, whereas dACE2 was not detected in bafilomycin- or IFNα-induced exosomes. However, dACE2 was found in low amounts in the cellular fraction of BALF from 50 patients. It did not correlate with COVID-19 severity, although dACE2 correlated positively with ACE2+ exosome proportions and ACE2 MFI on BAL-derived exosomes.
"High" ACE2+ exosome producers showed upregulation of genes involved in the CoV pathogenesis pathway, IFN and phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) signaling pathways and cytosolic PRR (pattern recognition receptor) activation, and downregulation of those involved in oxidative phosphorylation- and eIF2 (eukaryotic initiation factor 2) signaling-pathways. ACE2+ exosome addition to cell cultures led to dose-dependent reductions in SARS-CoV-2 N staining. Microscopy findings showed enhanced virion clustering and binding to ACE2 present on exosomes.
As a result of the study findings, defensosomes can potentially be used as anti-SARS-CoV-2 agents.