Cellular senescence is an irreversible cell cycle arrest that is induced in response to stress. Under stressed conditions, matrix remodeling enzymes and pro-inflammatory cytokines are produced, which are referred to as senescence-associated secretory phenotype (SASP).
In young individuals with physiological conditions, such as tumor suppression and wound healing, SASP aids in the recruitment of immune cells, which facilitate tissue restoration and the clearance of senescent cells. In older individuals, senescent cells accumulate due to the reduced functioning of the immune system and higher tissue damage.
To date, most senoylatic therapies have included small-molecule drugs that require repeated administration and poorly target the affected region. Comparatively, CAR T-cells require a single target antigen that is differentially expressed as compared to normal tissues. Additionally, CAR T-cells are single-administered ‘living drugs,’ which persist for many years and mediate their effects.
About the study
Previously, CAR T-cells have been shown to target the cell-surface protein uPAR and effectively deplete senescent cells. The current study examines whether CAR T-cells effectively and safely remove senescent cells in aged mice, thereby regulating health span.
Mice of both sexes, 8-12 weeks and 18-20 months of age, were included in the study. They were kept in group housing and pathogen-free conditions.
A 12-hour light/dark cycle was used and temperature and humidity conditions were standard. Aging mice consumed a normal diet, whereas a subset of mice consumed a high-fat diet (HFD).
For flow cytometry analysis, livers were dissociated and filtered, followed by the lysing of red blood cells. Single-cell ribonucleic acid (RNA) sequencing was performed, followed by expansion, isolation, and transduction of mouse T-cells. Complete blood measurements were noted and post-mortem analysis of multiple organs was performed.
Key findings
Senolytic cell therapies have been shown to mitigate symptoms related to physiological aging, including metabolic dysfunction. To this end, the proportion of uPAR-positive cells often rises as age progresses; therefore, both immune and non-immune uPAR-positive cells likely facilitate the senescence burden in aged tissues.
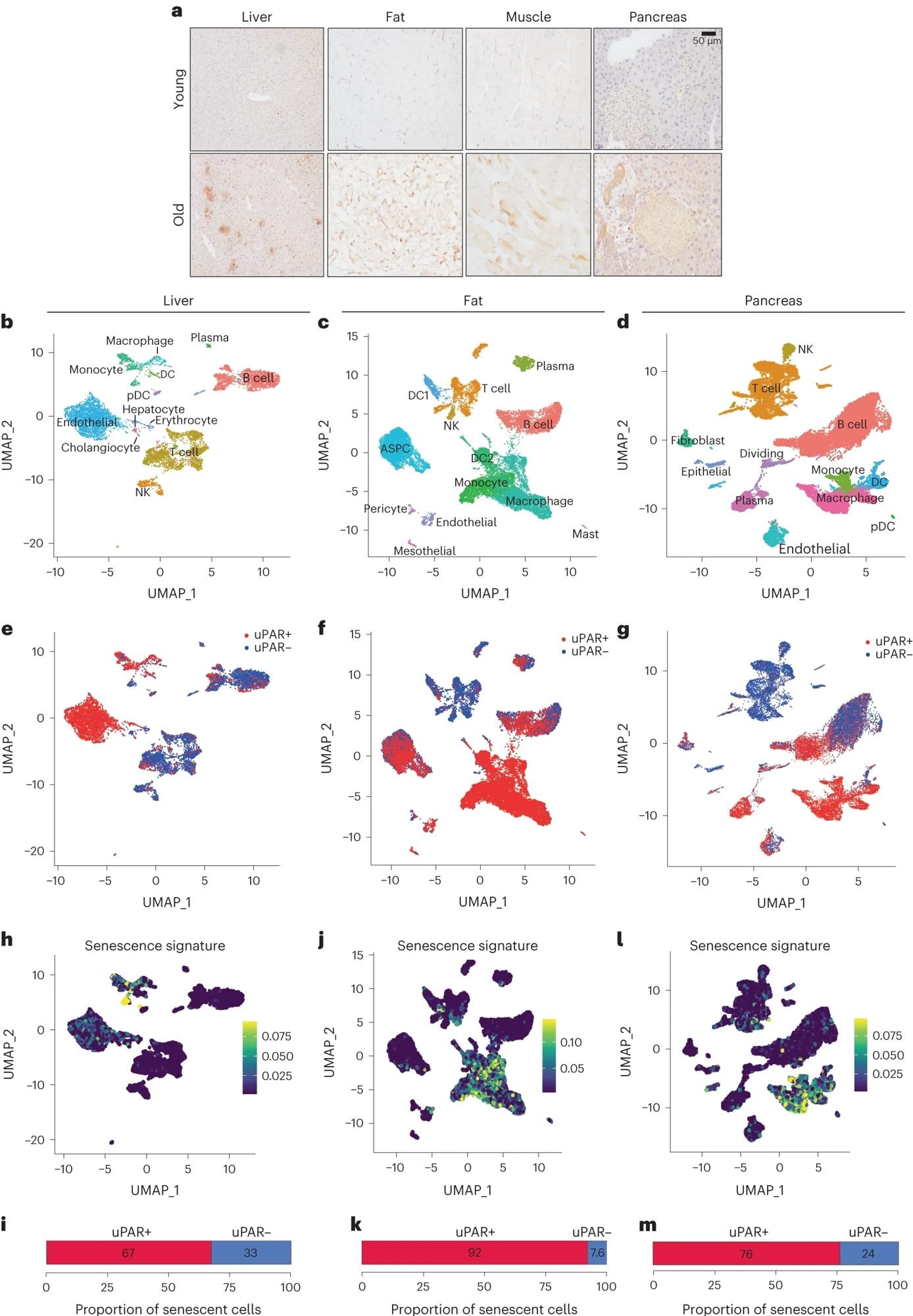
a, Immunohistochemical staining of mouse uPAR in liver, adipose tissue, muscle and pancreas from young (age 3 months) or old (age 20 months) mice (n = 3 per age). b–m, Single-cell analysis of uPAR expression and senescence. uPAR-positive and uPAR-negative cells were sorted from the liver, adipose tissue and pancreas of 20-month-old mice and subjected to single-cell RNA-seq by 10x chromium protocol (n = the sequencing of four mice where two females were combined into one replicate and two males were combined into another replicate). b, Uniform manifold approximation and projection (UMAP) visualization of liver cell types. c, UMAP visualization of adipose tissue cell types. d, UMAP visualization of pancreas cell types. e, UMAP visualization of hepatic uPAR-negative and uPAR-positive cell types. f, UMAP visualization of adipose uPAR-negative and uPAR-positive cell types. g, UMAP visualization of pancreatic uPAR-negative and uPAR-positive cell types. h,j,l, UMAP visualizations with senescence signature scores24 in each cell indicated by the color scale. i,k,m, Quantification of the proportion of uPAR-positive and uPAR-negative cells contributing to the respective senescence signature. h,i, Liver; j,k, adipose tissue; l,m, pancreas. Results are from one independent experiment (a–m). DC, dendritic cell; NK, natural killer; pDC, plasmacytoid dendritic cell; ASPC, adipose progenitor and stem cells.
The efficacy of uPAR CAR T-cells in removing uPAR-positive senescent cells was also highlighted. To this end, the efficacy of uPAR CAR T-cells is unrelated to tissue pathology or changes in the renal and hepatic parameters in aged mice.
The action of uPAR CAR T-cells was found to be linked to metabolic fitness and augmented glucose homeostasis in normally aging and HFD-feeding mice. Importantly, no toxicity was observed following the administration of uPAR CAR T-cells at recommended doses.
The potential of uPAR CAR T-cells to act prophylactically to mitigate diet-related and age-related metabolic decline was another striking observation. Moreover, uPAR CAR T-cells have long-lasting effects on senolytic methods based on small molecules. After a single administration, these cells impaired HFD-related or age-induced metabolic syndrome in mice administered with HFD or those that were treated during youth.
With respect to glucose tolerance, senolytic studies have identified the removal of senescent pancreatic beta cells. However, immune cell senescence could also have played a role.
Elimination of macrophages with senescent features has also been suggested to alleviate tissue decline in mice. This is consistent with the study observations, in which a proportion of uPAR-expressing macrophages coexpress transcriptional signatures associated with senescence and senescence-associated β-galactosidase (SA-β-gal).
Conclusions
The mechanism of action of small molecules is often poorly understood; however, senolytic CAR T-cells have a clear underlying mechanism due to the expression of specific surface antigens. This approach is associated with numerous advantages as compared to vaccination or small-molecule approaches, as cellular therapies regulate persistence through varied CAR designs and are equipped with certain safety switches.
In the future, cellular therapy could target these antigens to treat different phenotypes. The durability of the effects and persistence of uPAR-targeted CAR T-cells after a single administration augurs well for the senolytic CAR T-cell approach to treating chronic pathologies.
Journal reference:
- Amor, C., Fernandez-Maestre, I., Chowdhury, S., et al. (2024) Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction. Nature Aging; 1-14. doi:10.1038/s43587-023-00560-5