In a recent review published in the journal Frontiers in Nutrition, researchers from the United States of America discussed the clinical use of milk ladders in the management of immunoglobulin E (IgE)-mediated cow's milk allergy (CMA) among children. They explored the current evidence and benefits associated with this approach and provided recommendations to facilitate its safe clinical application.
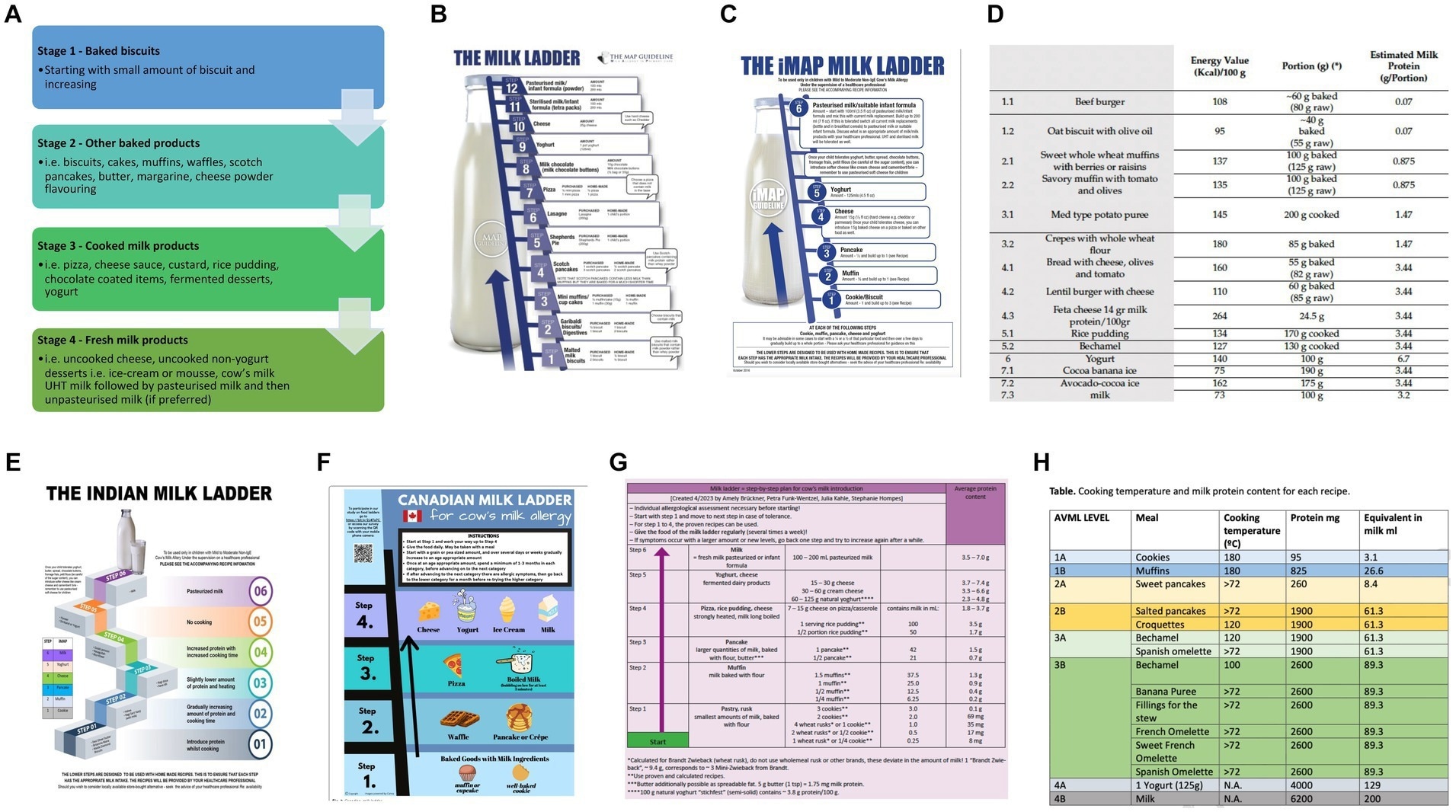 Current milk ladders (A) BSACI (B) MAP (C) iMAP (D) Mediterranean (E) Indian (F) Canadian (G) German (H) Spanish. Study: The future of cow’s milk allergy – milk ladders in IgE-mediated food allergy.
Current milk ladders (A) BSACI (B) MAP (C) iMAP (D) Mediterranean (E) Indian (F) Canadian (G) German (H) Spanish. Study: The future of cow’s milk allergy – milk ladders in IgE-mediated food allergy.
Background
Cow's milk allergy (CMA) is prevalent in children, with IgE and non-IgE mediated types diagnosed through clinical history, testing, and oral food challenges (OFCs). Management of this complex condition involves tailored avoidance, the use of hypoallergenic formulas, and the administration of emergency medications for anaphylaxis. Immunotherapy can increase tolerance but entails risks. Despite a favorable prognosis, many children continue to avoid cow's milk (CM) even after tolerating baked milk (BM) in OFCs. Improved standardized guidance for the home introduction of BM is needed. BM may also have a role in managing non-IgE mediated CMA, such as FPIES (short for food protein-induced enterocolitis syndrome) and EoE (short for eosinophilic esophagitis). The milk ladder approach, introduced in Ireland, has shown promise in safely introducing cow's milk at home for IgE-mediated CMA, improving management outcomes. In the present review, researchers discuss this approach in depth and provide recommendations for its clinical use in children with IgE-mediated CMA.
The basis for ladders
Food ladders, such as the milk ladder, are utilized in managing CMA by gradually introducing heated forms of milk to induce tolerance. These ladders exploit heat's effect on reducing the allergenicity of proteins in CM. The development of tolerance via ladders has been observed, particularly in limited-resource settings like Ireland. However, their home use carries inherent risks, including batch-to-batch variability in allergenic protein content and uneven heating within food items. Standardization and clear instructions are crucial for safe implementation. The benefits of food ladders include accelerating allergy resolution, expanding diet diversity, reducing healthcare costs, and lessening patient burdens. A previously published rostrum recommends the standardization of ladders, consideration of nutritional value, acceptance by patients, and accommodation of cultural practices. Further studies are needed to fully characterize the risks and benefits of milk ladder use.
Assessing ladders
When evaluating the different CM ladders currently accessible, it's essential to assess factors concerning the ladder's design, the specific patient and family involved, the healthcare environment they navigate, and how the ladder influences the patient's overall nutrition.
Ladder design
For effective use in clinical practice, CM ladders for IgE-mediated CMA must involve a gradual increase in CM protein content with reduced denaturing as the ladder progresses. The optimal starting dose and the rate of dose increase need to be considered. Foods in one step should contain the same amount of CM protein. Ladders should also include simple and straightforward recipes for improved adoption by families. The ladder's milk protein content should be verified in the lab. Nutritional content, palatability, and cultural appropriateness are crucial considerations for the successful implementation of ladders for CMA management.
Patient selection
Safe implementation of milk ladders requires careful consideration of patient-specific factors, including unpredictable reactions, modifying factors, and family dynamics. Considering socio-economic constraints, providers must assess the family's willingness and ability to procure, prepare ladder foods, and respond to allergic reactions.
Healthcare system
Healthcare system dynamics influence the feasibility of using ladders for IgE-mediated allergy, especially in regions with limited subspecialty access. Home ladders may serve as a resource-efficient alternative to observed food challenges, particularly in settings with constrained pediatric allergy resources like Ireland. However, considerations regarding healthcare costs, availability of allied healthcare professionals, and medicolegal aspects warrant careful evaluation by providers.
Nutrition
Providers should also consider the effects of using a ladder beyond allergen exposure. Ladder use may enhance diet diversity, potentially improving the quality of life for children and families by reducing dietary restrictions. This could also lead to improved growth parameters in food-allergic children, necessitating further research to confirm these potential benefits.
Benefits of ladder use beyond allergen-introduction
Food ladders for IgE-mediated CMA offer benefits beyond allergen introduction, including increased food variety, reduced need for label reading, decreased food-related anxiety, and potential financial savings. These ladders may promote tolerance and improve socialization, diet normalization, and nutritional intake, particularly fiber.
Although various factors influence ladder safety, adherence to calculated progression and allergenicity testing are crucial for ensuring the safety of their use.
Conclusion
In conclusion, further research is needed to optimize ladder design and demonstrate the effectiveness of milk ladders. However, they may be judiciously used in selected patients to bring about positive outcomes, thus shaping the management of Ige-E-mediated CMA. Future studies in this regard may also involve considering the immunomodulatory aspects of children's dietary intake, including fat, sugar, and fiber content.