A landmark study shows that when babies start walking isn't just developmental, it’s in their DNA. Genetic variants not only shape motor timing but also link to cognitive traits and brain structure.
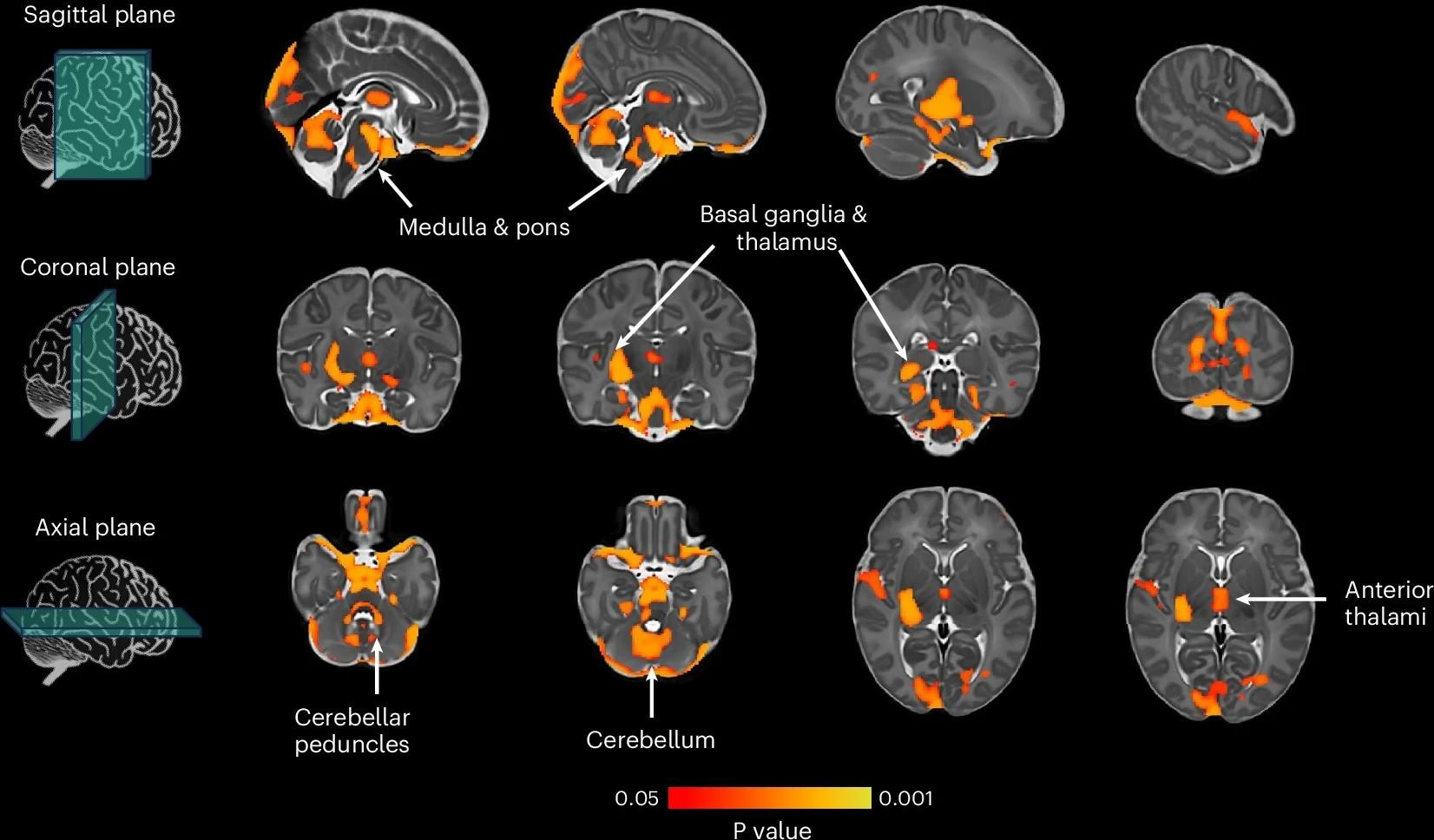 Thresholding t-statistic image at t > 0.95 (two-sided statistical test). Significant voxels were overlaid on the 40-week neonatal brain template in sagittal, coronal and axial planes. White arrows indicate significant brain structures involved in motor control. N = 264. Study: Genome-wide association meta-analysis of age at onset of walking in over 70,000 infants of European ancestry
Thresholding t-statistic image at t > 0.95 (two-sided statistical test). Significant voxels were overlaid on the 40-week neonatal brain template in sagittal, coronal and axial planes. White arrows indicate significant brain structures involved in motor control. N = 264. Study: Genome-wide association meta-analysis of age at onset of walking in over 70,000 infants of European ancestry
A recent study published in the journal Nature Human Behavior performed a genome-wide association study (GWAS) meta-analysis of age at onset of walking (AOW).
The onset of walking in early childhood is a robust clinical marker of brain and behavioral development. Moreover, walking is an exclusive, informative milestone for atypical and typical development. The inability to walk independently by 18 months is a screening criterion to refer to a pediatrician for further assessment because delayed walking might indicate a motor-specific or generalized issue.
However, historical data suggest that only a minority of late walkers may have an underlying developmental disorder or neurological abnormality. As such, late walkers may reflect an extreme of typical variation or a clinically meaningful condition with a later onset age. AOW is a complex trait shaped by various factors, such as body dimensions, gestational age, neural maturation, year of birth, nutrition, opportunity to practice, and cultural context.
Many of these factors influence the function and structure of brain areas implicated in motor control, including the basal ganglia, cerebellum, and cortex. Dysfunctions in these regions result in movement disorders. Nevertheless, it remains unclear what the causal influences underlying AOW variability are and whether they are associated with later health, cognitive, and neurodevelopmental outcomes.
The study and findings
In the present study, researchers performed a GWAS meta-analysis of AOW in 70,560 infants from four European-ancestry cohorts: the United Kingdom Medical Research Council National Study for Health and Development (NSHD), the Lifelines Multigenerational Prospective Population-based Birth Cohort study, the Netherlands Twin Register (NTR), and the Norwegian Mother, Father, and Child Cohort Study (MoBa).
This revealed 2,525 genome-wide significant single-nucleotide polymorphisms (SNPs). Of these, 11 were independent loci with one lead variant per locus, which remained significant after conditioning on other significant SNPs within the same chromosome. The most strongly associated SNPs, rs7956202 and rs16952251, were located on chromosomes 12 and 16, respectively. SNP-based heritability of AOW was 24.13%.
The study found that approximately 11,900 SNPs account for about 90% of the SNP-based heritability for AOW, highlighting the trait’s high polygenicity.
No significant genome-wide heterogeneity was observed between cohorts. Further, the genome-wide SNPs were mapped to 233 genes based on expression quantitative trait loci (eQTLs), chromatin interaction information, and genomic position. The team also evaluated whether these prioritized genes had differential expression in the brain across BrainSpan developmental stages and Genotype-Tissue Expression (GTEx) tissues.
As such, they noted a significant downregulation of differentially expressed genes (DEGs) in various tissues, including the brain and the heart, and DEG upregulation in fibroblasts. However, the enrichment of upregulated or downregulated DEGs across BrainSpan developmental stages was not significant. AOW-related gene sets were enriched in gene ontology (GO) generation of neurons and neurogenesis pathways. The Multi-marker Analysis of GenoMic Annotation (MAGMA) gene-based test performed on meta-GWAS summary statistics revealed that 50 genes were associated with AOW.
Thirteen of these genes were associated with intellectual disability, and seven were associated with autism. The team also found that the expression of AOW-associated genes was significantly enriched in 19–24 post-conceptional weeks. Next, the researchers tested the enrichment of the AOW meta-GWAS signal by functional genomic annotation.
This revealed significant enrichment of AOW heritability in genomic regions conserved in mammals, primates, and vertebrates. Additionally, the team assessed whether AOW heritability was enriched in specific cell types and noted significant enrichment in the brain, especially in the cortex, cerebellum, basal ganglia, and amygdala. A polygenic score (PGS) was calculated based on meta-analyses of all samples, leaving out either NTR, Lifelines, or NSHD cohorts.
In the Lifelines cohort, the PGS from the meta-GWAS of other cohorts was significantly associated with AOW. Likewise, the PGS was significantly associated with AOW in the NSHD and NTR cohorts. Within the largest cohort, MoBa, cross-validation was used to assess the predictive value of the PGS.
Next, the team examined genetic correlations between AOW and select neurodevelopmental, physical health, cortical, psychiatric, and cognitive phenotypes.
AOW exhibited a negative genetic correlation with childhood and adult body mass index (BMI) and attention deficit/hyperactivity disorder (ADHD). The negative genetic correlation between AOW and ADHD may reflect that children with higher ADHD genetic liability exhibit higher early motor activity or shorter attention spans, which could facilitate earlier walking. Besides, it showed a positive genetic correlation with cognitive phenotypes and folding index. While AOW showed a genetic correlation with self-reported walking pace in adults, it was no longer significant after multiple testing. Further, the team explored whether the PGS for AOW was associated with differences in brain volume and gyrification at birth.
They found a significant positive correlation between the PGS for AOW and regional brain volume in the right posterior thalamus, right basal ganglia, superior temporal sulcus, bilateral anterior thalami, pons, primary visual cortex, medulla, and bilateral cerebellum and cerebellar peduncles in neonatal brains. Moreover, the AOW PGS was significantly positively associated with the gyrification index in both hemispheres in neonatal brains.
The study also demonstrated, using within-family (twin) analyses, that the polygenic score effect on AOW is largely a direct genetic effect rather than due to confounding from population stratification, assortative mating, or passive gene–environment correlation.
Notably, one of the loci identified (in the gene RBL2) is also associated with a rare neurodevelopmental disorder characterized by delayed or absent walking, providing further biological plausibility to the findings.
The researchers also found substantial genetic overlap between AOW and traits such as cognitive performance and educational attainment, with more than half of shared genetic variants showing concordant effects (i.e., influencing both traits in the same direction), though nearly half had discordant effects. This highlights the complexity of genetic relationships between early motor milestones and later cognitive abilities.
Conclusions
The study illustrated that AOW is a heritable polygenic trait with significant etiological links to later health outcomes. Eleven genome-wide significant loci associated with AOW were identified, one of which colocalized with an eQTL. Besides, the high variability in AOW is partly due to common genetic variation, with nearly one-fourth of the variability explained by common genetic variants. The identified genetic variants plausibly contribute to individual variability in motor behavior.
The authors note that their study was limited to cohorts of European ancestry, and some measurements of AOW relied on parent recall several years after infancy, which could introduce measurement error. Future research in more diverse populations and with more precise phenotyping is needed.
Journal reference:
- Gui A, Hollowell A, Wigdor EM, et al. Genome-wide association meta-analysis of age at onset of walking in over 70,000 infants of European ancestry. Nature Human Behavior, 2025, DOI: 10.1038/s41562-025-02145-1, https://www.nature.com/articles/s41562-025-02145-1