Scientists harness a new-to-nature reaction in E. coli to turn discarded plastics into life-saving medicines, charting a sustainable path for chemical manufacturing.
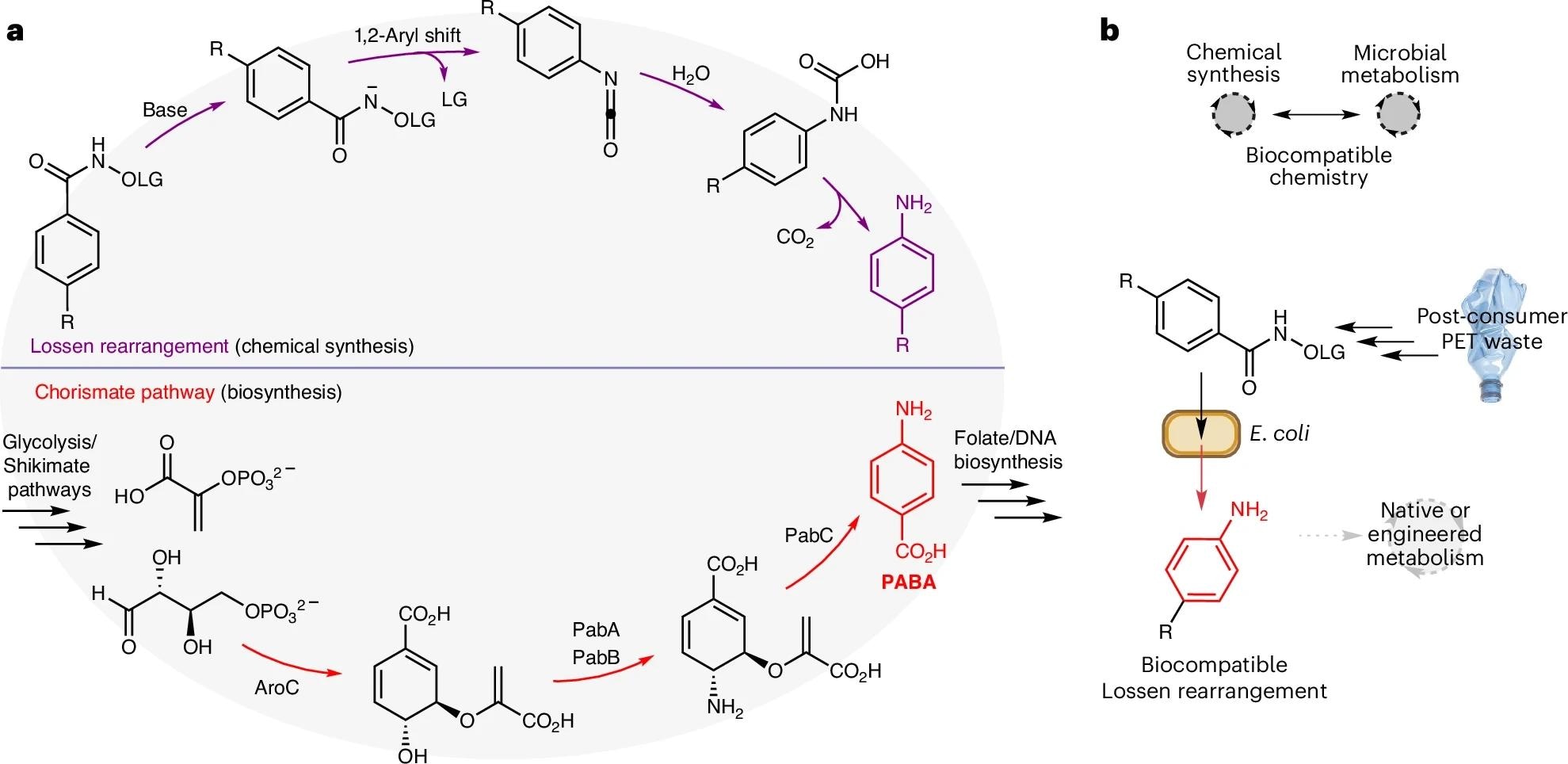 a, A comparison of strategies for C–N bond formation via Lossen rearrangement in synthetic organic chemistry or via chorismate pathways in cellular metabolism. b, The proposed merging of non-enzymatic Lossen rearrangement chemistry with cellular metabolism for sustainable synthesis and the bio-upcycling of plastic waste. LG, leaving group.
a, A comparison of strategies for C–N bond formation via Lossen rearrangement in synthetic organic chemistry or via chorismate pathways in cellular metabolism. b, The proposed merging of non-enzymatic Lossen rearrangement chemistry with cellular metabolism for sustainable synthesis and the bio-upcycling of plastic waste. LG, leaving group.
In a recent study published in the journal Nature Chemistry, researchers demonstrated a first-of-its-kind experiment in which they enabled Escherichia coli bacteria to catalyze a classic yet novel chemical reaction: the catalytic transformation of activated acyl hydroxamates into amines.
Their experiment marks a breakthrough in the relatively nascent field of biocompatible reactions. It allowed researchers to leverage the Lossen rearrangement, a synthetic organic chemistry catalytic reaction new to nature, to convert plastic (polyethylene terephthalate [PET]) waste into paracetamol. By blending synthetic chemistry with living systems, the study pioneers a new wave of biomanufacturing, where microbes recycle our waste and give us life-saving drugs.
Background
The global biotechnology machinery makes ample use of microbes, most notably Escherichia coli, as workhorses for the cheap, efficient, and large-scale manufacture of several valuable chemicals. Unfortunately, traditional biotechnology is limited in its ability to manipulate microbes' genetic toolkits, severely limiting the scope of their applications. Several chemical reactions, such as the Lossen rearrangement, remain restricted to synthetic chemistry labs and their associated scalability drawbacks.
To address this limitation and expand the influence of biotechnology, a relatively novel concept termed 'biocompatible chemistry' is rapidly gaining momentum. The concept combines human-directed non-enzymatic organic reactions and natural cellular metabolism, greatly expanding the raw materials microbes can consume and the products they can produce.
While biocompatible chemistry could, in theory, allow genetically engineered microbes to convert garbage into biofuels or even pharmaceuticals, the complex challenge of achieving non-toxic, efficient chemistry under physiological conditions must be met. So far, achieving this delicate balance has remained a significant challenge.
About the study
In the present study, researchers discovered that phosphate ions present in standard bacterial growth media can catalyse the Lossen rearrangement under biologically compatible conditions. Described in 1872 by Wilhelm Lossen, this hitherto synthetic-chemistry-lab-restricted experiment involves the phosphate-catalysed rearrangement of a phenyl hydroxamate ester into a primary amine product.
To reproduce Lossen rearrangements in living cells, researchers first synthesized an activated hydroxamate substrate carrying a para-carboxyl group. In aqueous M9 media at 37 °C, phosphate in the growth medium catalyzes this substrate into para-aminobenzoate (PABA), an essential precursor for folate biosynthesis.
They tested the setup using auxotrophic E. coli strains lacking pabA/B (ΔpabB or ΔpabA/B) or aroC genes, making them unable to produce PABA and thus unable to grow. With the Lossen substrate added, bacteria resumed growth, a process termed 'auxotroph rescue'. This suggests that the bacteria can now carry out the Lossen reaction and use that product as a nutrient source, serving as a clear functional readout that the reaction successfully integrated with E. coli metabolism.
To demonstrate the application potential of this novel E. coli strain, researchers conducted two successive experiments: 1. PET-derived substrate, and 2. Paracetamol synthesis. Researchers first chemically processed a polyethylene terephthalate (PET) bottle into a hydroxamate Lossen precursor outside the cell. They then grew a nutrient-starved culture of their engineered E. coli on their Lossen precursor, observing restored growth (at a rate of approximately 0.33 h⁻¹), demonstrating plastic-to-nutrient conversion.
Finally, they used genetically modified E. coli strains expressing O₂- and NADH-dependent aminobenzoate hydroxylase (ABH60) and acetyl-CoA-dependent arylamine N-acyltransferase (PANAT) genes, sourced from a fungus and another bacterium, respectively, to convert their Lossen precursor into para-hydroxyacetanilide (paracetamol). Initial attempts with a single engineered strain led to the formation of unwanted side products; the researchers addressed this by developing a more efficient two-strain system, with each strain performing one step of the conversion.
Study findings
This study marks a milestone in biocompatible chemistry research, demonstrating that chemically synthesized non-enzymatic organic compounds can be integrated into the natural world and processed using preexisting host metabolism, substantially expanding the scope of tomorrow's biotechnology. Its findings revealed that the Lossen rearrangement, a chemical reaction previously restricted to specialized chemistry labs, was achievable under routine, aqueous, physiological conditions and non-toxic in vivo.
The study identified auxotrophic E. coli strains capable of converting a custom-synthesized Lossen substrate into growth-promoting nutrients (PABA), confirming the integration of Lossen rearrangement into the bacteria's cellular machinery.
The study further revealed that these engineered bacteria were capable of not only degrading PET waste (bioremediation) but also of their genetically enhanced subvariants (ABH60 and PANAT-expressing strains) converting PET waste into paracetamol, the World Health Organization's (WHO) primary recommended first-line intervention against fever and pain.
Finally, the study confirmed that this system functioned similarly across a range of Lossen substrates and reaction targets, hinting at a generalizable platform for non-native chemical transformations in live cells.
Conclusions
The present study highlights the potential of biocompatible chemistry research in revolutionising tomorrow's chemical production. It demonstrates a novel strain of E. coli bacteria capable of combining human ingenuity with its natural cellular machinery to achieve a Lossen rearrangement. It channels the resulting products into growth and pharmaceutical production, even from plastic (PET) waste.
This research blurs the line between chemistry and biotechnology, offering a novel route to upcycle materials and synthesize value-added compounds. While this process is currently a proof-of-principle and yield optimization and pathway tuning remain challenges, this work lays a foundation for sustainable, cell-based systems that merge abiotic reactions with metabolism.