New research reveals how GLP-1 medications are reshaping the treatment landscape for conditions affecting the heart, kidney, liver, and more, hinting at a new era in chronic disease management.
 Commentary: The expanding benefits of GLP-1 medicines. Image Credit: Alexander_P / Shutterstock
Commentary: The expanding benefits of GLP-1 medicines. Image Credit: Alexander_P / Shutterstock
In a recent commentary article published in the journal Cell Reports Medicine, authors Maria J. Gonzalez-Rellan, Daniel J. Drucker, of Mt. Sinai Hospital, Toronto, Canada summarize and elucidate evidence that glucagon-like peptide-1 (GLP-1) receptor agonists (RAs), initially developed for the treatment of type 2 diabetes and weight management, demonstrate a surprisingly broad range of clinical benefits.
This evidence, drawn from several major clinical trials, reveals that GLP-1 RAs like Semaglutide and Tirzepatide can significantly reduce the risk of cardiovascular events, chronic kidney disease, and liver disease while improving conditions like sleep apnea and osteoarthritis.
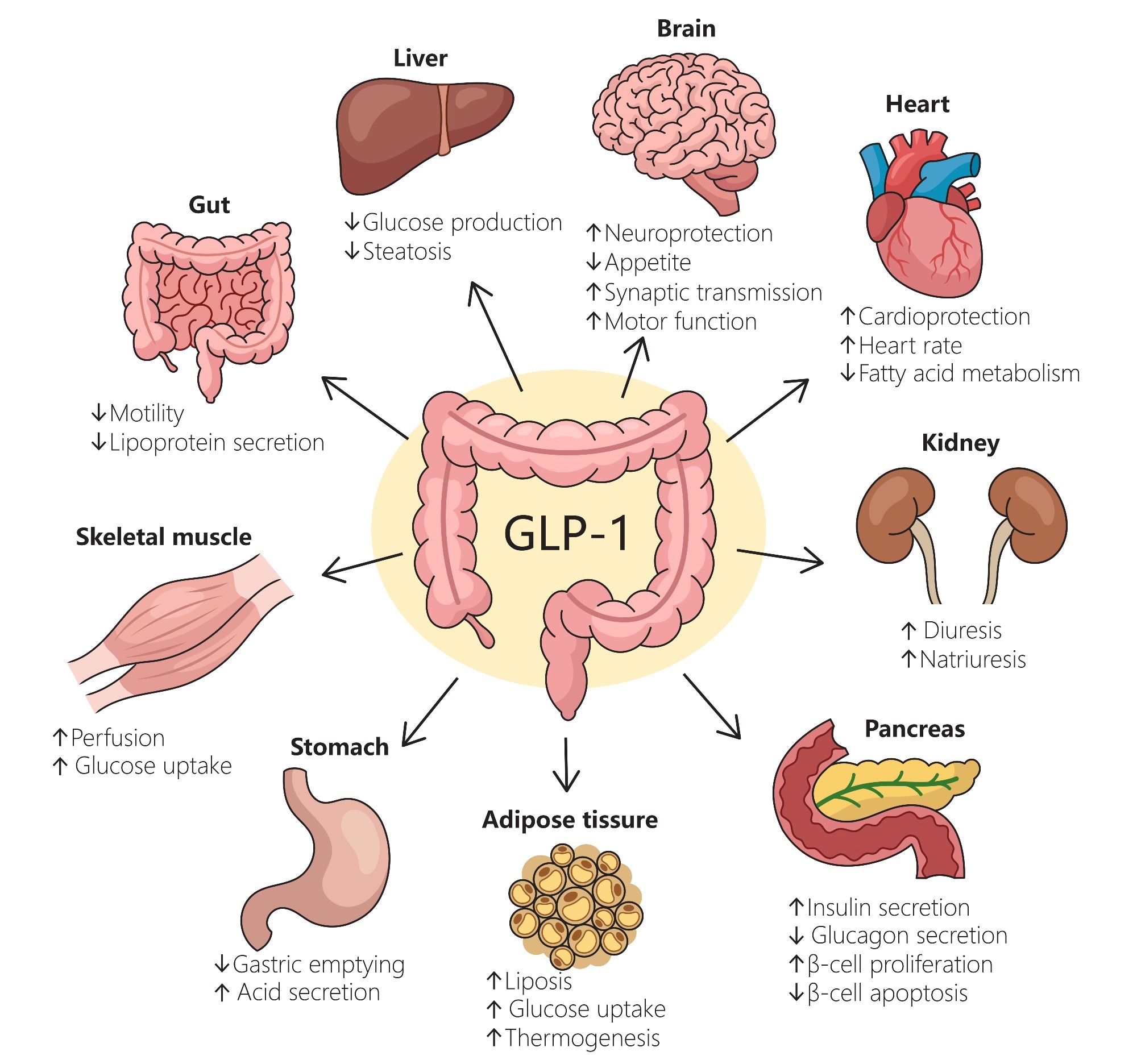
Mechanistic investigations highlight that GLP-1 RAs have not just weight loss, but also anti-inflammatory and metabolic properties, suggesting their efficacy against several chronic conditions. The review notes, however, that the degree to which these benefits are independent of weight loss may vary by condition, and further research is needed to elucidate these mechanisms fully.
Background
Glucagon-like peptide-1 (GLP-1) receptor agonists (RAs) are a class of medications initially developed to treat type 2 diabetes (T2D) and obesity. They regulate blood sugar and appetite by mimicking the action of the naturally occurring gut hormone GLP-1, a modulator of insulin secretion, glucagon, stomach emptying, and satiety signalling.
Semaglutide (marketed as 'Ozempic' and 'Wegovy') and the dual-agonist Tirzepatide ('Mounjaro' and 'Zepbound') are some of today's most talked-about medications, given their unprecedented clinical efficacies across both weight loss and T2D management.
Surprisingly, a growing number of clinical studies demonstrate that these drugs (and other GLP-1 RAs) may have benefits that extend far beyond their original purpose, with their effects spanning the heart, kidneys, liver, and more.
Recent evidence also suggests potential benefits in peripheral artery disease, which was studied in the STRIDE trial, and neurodegenerative disorders, such as ongoing research in Alzheimer’s disease (EVOKE and EVOKE+ trials). However, efficacy in these latter indications remains under investigation.
About the review
This review aims to simultaneously promote GLP-1 RA research and enhance users' understanding by synthesizing evidence from recent landmark trials, thereby providing a comprehensive picture of the potential of these drugs as more than just metabolic modulators.
It provides a comprehensive and up-to-date overview of the broad therapeutic landscape of GLP-1 RAs by consolidating the findings from multiple large-scale, randomized controlled trials (n = 15 publications).
Key drug-disease associations covered include: 1. Cardiovascular outcomes (e.g., SELECT and SOUL trials), 2. Chronic kidney disease (CKD) (e.g., FLOW), 3. Heart failure with preserved ejection fraction (HFpEF) (e.g, STEP-HFpEF and SUMMIT), 4. Metabolic dysfunction-associated steatohepatitis investigations (e.g, ESSENCE, SURVODUTIDE in MASH, and Tirzepatide in MASH), 5. Obstructive sleep apnea (e.g., SURMOUNT-OSA), and 6. Osteoarthritis (e.g., STEP 9). The review also discusses emerging data in peripheral artery disease (e.g., STRIDE trial).
Review findings
The review emphasizes that the benefits of GLP-1 medicines are multi-organ and driven by complex mechanisms. For example, in the SELECT trial (17,600 patients with cardiovascular disease and obesity but without T2D), weekly subcutaneous Semaglutide (2.4 mg) reduced the risk of major adverse cardiovascular events (MACE – heart attack, stroke, or cardiovascular death) by 20% compared to placebo (HR 0.80; p < 0.001). Critically, this benefit was not solely dependent on the amount of weight lost.
CKD benefits were similar, with the FLOW trial (3,500 patients with T2D and chronic kidney disease), Semaglutide (1 mg weekly) reduced the risk of kidney failure events and death from kidney- or cardiovascular-related causes by 24% (HR 0.76; p < 0.001), leading the FDA to expand its approval (January 2025) to include reducing the risk of kidney disease progression.
The SURMOUNT-OSA trials revealed that Tirzepatide dramatically reduced obstructive sleep apnea severity by finding that the drug decreased case patients' apnea-hypopnea index (AHI), a measure of breathing interruptions, by an estimated 20–24 events per hour more than controls, depending on whether patients were using positive airway pressure (PAP) therapy at baseline. In the PAP group, the mean reduction from baseline was as much as 29.3 events per hour.
Osteoarthritis was no different, with the STEP 9 trial finding that Semaglutide improved patients' physical function and reduced their knee pain intensity (scores).
Most strikingly, multiple trials have now shown that GLP-1 agonists can lead to the resolution of MASH, a severe form of fatty liver disease, without worsening of liver fibrosis, which is a common adverse outcome seen with some current medications. However, typical gastrointestinal side effects associated with GLP-1 agents remain.
Additionally, the review highlights the STRIDE trial in peripheral artery disease, where Semaglutide improved maximum walking distance in people with T2D and PAD, an effect correlated in part with weight loss.
Conclusions
The present review goes so far as to suggest that GLP-1 medicines represent a watershed moment in treating chronic diseases, with their therapeutic reach extending well beyond their creators' expectations. The drugs are now known to demonstrate potent benefits for the cardiovascular, renal, and hepatic systems, with notable effects that are partly independent of their simultaneous effects on weight loss and type 2 diabetes. However, the extent to which these benefits are truly independent of weight loss may differ by condition, and more research is needed to clarify these relationships.
Preliminary mechanistic investigations suggest that GLP-1 medicines exert direct anti-inflammatory effects, reducing biomarkers such as C-reactive protein (CRP) and tumor necrosis factor-alpha (TNF-α). Future research should aim to unravel the precise molecular mechanisms underlying these weight-independent benefits, enabling the development of new therapies that harness these anti-inflammatory pathways and potentially bypass the limitations of current interventions.
Encouragingly, ongoing trials, such as EVOKE and EVOKE+, are already exploring this potential for early Alzheimer's disease. The review cautions that effective dosing, duration, and efficacy for non-metabolic and neuropsychiatric indications, including Alzheimer’s disease and substance use disorders, remain to be established.
While the breadth of GLP-1 medicines’ clinical impact is striking, the review maintains a scientific and measured tone, highlighting the importance of further rigorous investigation to understand and optimize these therapies for diverse chronic conditions fully.