A radically simple pill could change the future of IBD care; just one dose can turn signs of inflammation into a clear, visual cue right at home.
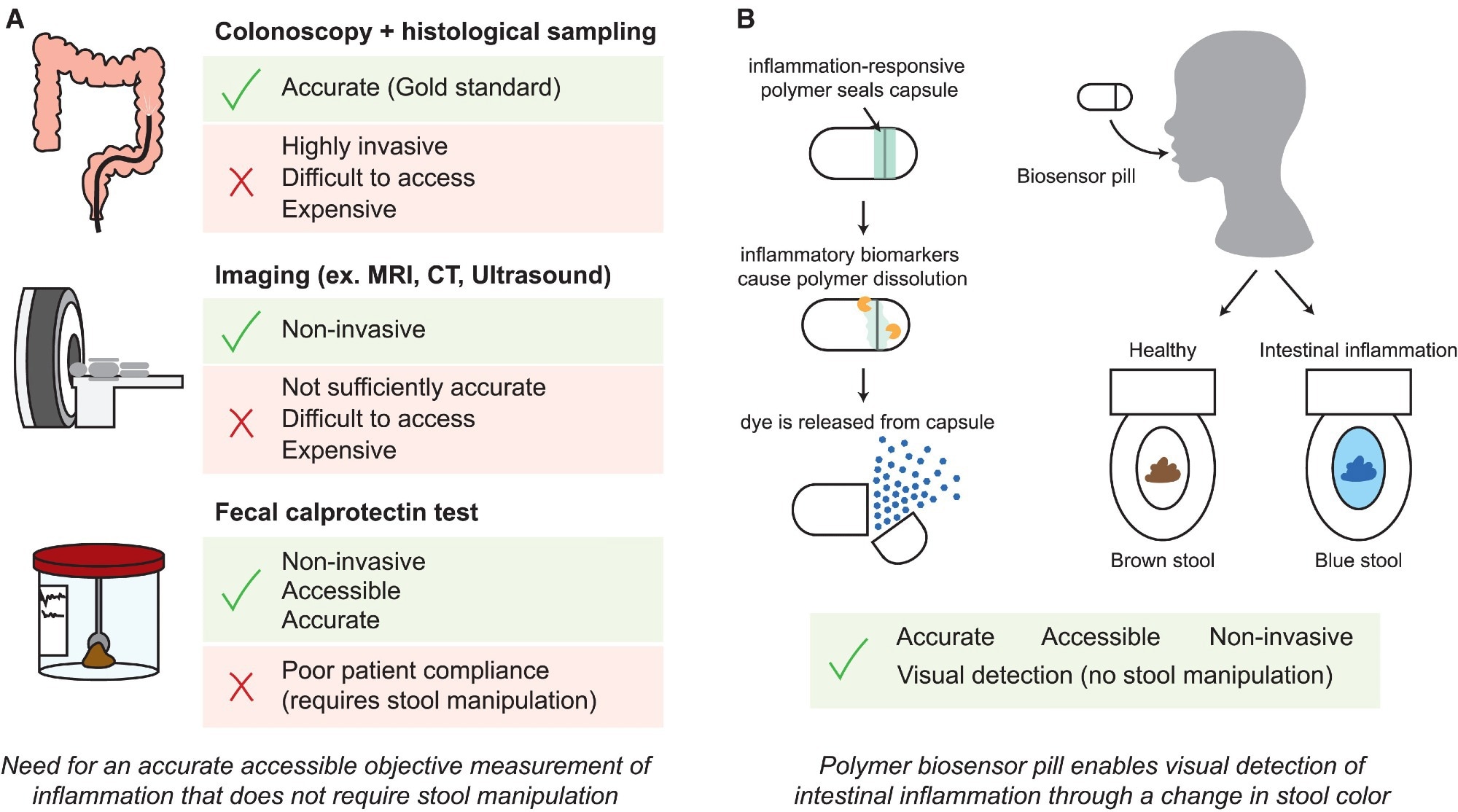 Schematic of modalities for inflammation monitoring in IBD. Study: A radically simple, ingestible colorimetric biosensor pill for cost-effective, non-invasive monitoring of intestinal inflammation
Schematic of modalities for inflammation monitoring in IBD. Study: A radically simple, ingestible colorimetric biosensor pill for cost-effective, non-invasive monitoring of intestinal inflammation
In a recent study published in the journal Device, researchers developed an ingestible biosensor pill for monitoring intestinal inflammation.
Inflammatory bowel diseases (IBDs) affect more than seven million people worldwide. IBD is characterized by episodic inflammation in the colon and small intestine. IBD lacks a curative treatment, but effective therapies are available to help attain and maintain remission. Current options for monitoring inflammation are suboptimal.
Colonoscopy remains a critical aspect of IBD diagnosis and monitoring. Still, the invasiveness, patient discomfort, high costs, and requirement of specialized medical facilities and personnel make it unviable for frequent disease surveillance. Critically, current fecal tests, such as calprotectin, suffer from ~50% patient non-compliance due to aversion to stool handling.
Recently, photosensitive, pH-sensitive, and bacterial sensor-based pill devices have been developed to monitor changes in the gastrointestinal (GI) environment associated with inflammation. However, they are complex and require genetic or electrochemical circuits, which increase costs and reduce their likelihood of clinical adoption.
The study and findings
In the present study, researchers developed a pill for reactive oxygen species (ROS)-responsive inflammation monitoring (PRIM), an ingestible biosensor device for non-invasive, at-home detection of intestinal inflammation that eliminates the need for fecal handling.
First, a ROS-responsive polymer was synthesized by modifying dextran with phenylboronic ester functional groups. Phenylboronic esters undergo selective degradation in the presence of hydrogen peroxide (H₂O₂), a type of ROS elevated 10–100× in IBD patients. The ROS-responsive dextran polymer remained insoluble in water; however, exposure to H₂O₂ caused the polymer to disintegrate, highlighting its responsiveness to ROS.
Scanning electron microscopy (SEM) revealed cracks in coatings made from ROS-responsive dextran polymer. Exposure to water resulted in partial flaking of the polymer layer, but no polymer was observed following H₂O₂ exposure. Therefore, after discovering that residual phenylboronic ester acted as an accidental plasticizer, acetyl tributyl citrate (ATBC, 44% w/w), a biodegradable plasticizer, was intentionally used as an additive to enhance the mechanical properties. This resulted in smooth coatings that remained intact after 24 hours of water exposure. H₂O₂ exposure visibly eroded coatings, confirming the retention of ROS-responsive properties.
The PRIM device was composed of a 3D-printed pill cap and body with an O-ring sealing the body, and was designed to be the size of a 00 capsule (common in OTC medications). A common food dye, Brilliant Blue FCF, was used to fill the body of the PRIM device; this dye remains unabsorbed in the digestive tract, altering fecal color while also diffusing into toilet water for easier detection.
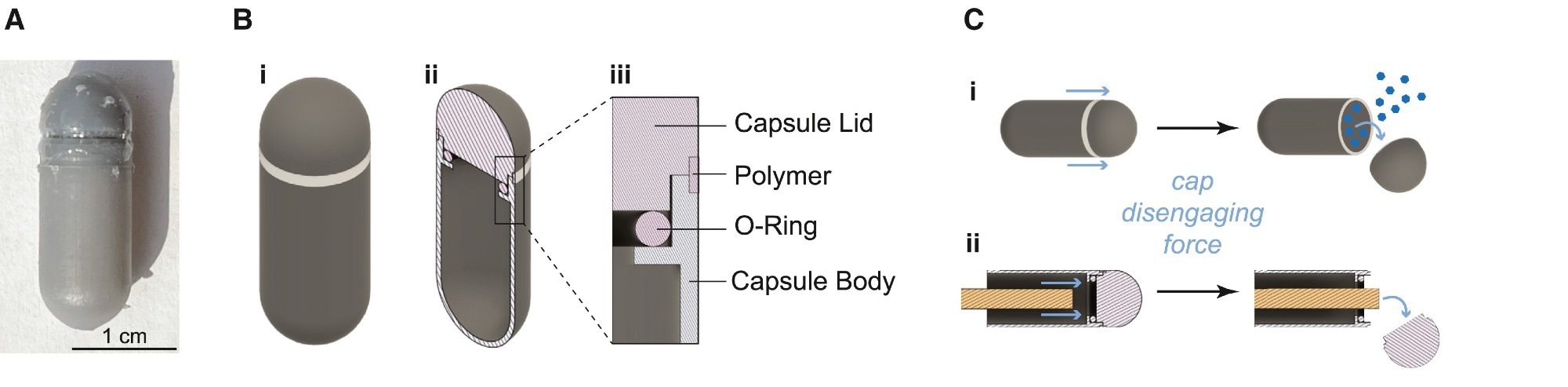 (A) PRIM device sealed with ROS-responsive polymer adhesive coating. (B) Schematic of (i) the 00 capsule-sized PRIM device, (ii) cross-section of the PRIM device showing the capsule lid and the hollow chamber that is filled with dye, and (iii) inset of the cross-section showing how an O-ring is used to form a seal between the capsule body and lid and where ROS-responsive dextran is filled into a notch to adhere the lid and body together. (C) Schematic of (i) PRIM device mechanism design for cap detachment and dye release and (ii) experimental measurement of cap disengaging force using a rod to push off the cap.
(A) PRIM device sealed with ROS-responsive polymer adhesive coating. (B) Schematic of (i) the 00 capsule-sized PRIM device, (ii) cross-section of the PRIM device showing the capsule lid and the hollow chamber that is filled with dye, and (iii) inset of the cross-section showing how an O-ring is used to form a seal between the capsule body and lid and where ROS-responsive dextran is filled into a notch to adhere the lid and body together. (C) Schematic of (i) PRIM device mechanism design for cap detachment and dye release and (ii) experimental measurement of cap disengaging force using a rod to push off the cap.
There was a notch for adding ROS-responsive dextran at the junction between the pill body and device cap to adhere the body and cap together. Cap detachment upon exposure to ROS releases the dye, indicating the presence of higher ROS levels via a threshold mechanism requiring sustained inflammation.
Furthermore, the adhesive strength of the ROS-responsive dextran was measured as the force required to disengage the cap from the PRIM device. The cap disengagement force after H₂O₂ exposure for 24 hours was lower than that after exposure to phosphate buffer. Higher ROS levels during inflammation could degrade the polymer adhesive and detach the cap, while healthy baseline levels do not. In vitro experiments showed that the dye was not released at low levels of H₂O₂ (≤ 1 mM) even after 72 hours and remained stable during simulated GI transit (up to 72 hours). However, at higher concentrations (50 mM), all devices underwent polymer adhesive degradation within 48 hours, releasing the dye within 72 hours – a finding that aligns with the slower transit times observed in IBD patients.
The team also tested the stability of the PRIM device in several in vitro tests that simulate healthy GI environments, including variable pH, digestive mechanical forces, and digestive enzymes. All PRIM devices were intact after incubation in neutral, acidic, and alkaline pH conditions and exposure to simulated chyme, gastric acid, and gastric fluid for 72 hours. All devices were also intact following continuous mechanical agitation at physiologically relevant timescales.
Finally, the PRIM device was miniaturized as a prototype for in vivo testing in a rat model of colitis. Co-administration studies confirmed blue dye detection remained unambiguous even with dietary pigments like beet-derived betanin. Rats were administered three miniaturized PRIM devices, and colitis was induced by administering dextran sulfate sodium (DSS) for a week.
Three additional devices were administered after induction of colitis. The team observed that 78% of PRIM devices activated in rats with colitis compared to 28% in healthy rats (controls). Rats with activated devices produced feces of bright blue color before returning to normal colors after 48 hours. Specificity improved to 92% (in two tests) or 100% (in three tests) with sequential administrations.
Conclusions
In sum, the study developed an ingestible, accessible, and minimally invasive device for detecting upregulated ROS markers associated with intestinal inflammation. The PRIM device was sealed using a polymer adhesive, ROS-responsive dextran, that selectively degrades upon H₂O₂ exposure to release the cargo dye.
The adhesive remained stable in the absence of H₂O₂, and no dye was released at low levels of H₂O₂. In the in vivo model of colitis, the miniaturized PRIM device revealed the presence of inflammation by releasing the dye, resulting in colored feces. It demonstrated 72% specificity and 78% sensitivity in detecting colitis.
Production costs are estimated at $0.38 per device at scale. Further testing and optimization of the full-sized (human) PRIM in large animals should improve its specificity and sensitivity. Future work will address the potential for rapid transit during diarrhea (potentially resulting in false negatives) through repeat dosing and validate the findings against chronic inflammation models.
Overall, PRIM is a promising, cost-effective device for the frequent, non-invasive monitoring of intestinal inflammation, overcoming patient compliance barriers and demonstrating robustness to dietary confounders.