From endothelial repair to anti-inflammatory effects, naringin demonstrates powerful heart-protective actions before reaching clinical trials.
 Systematic Review: Endothelial and Cardiovascular Effects of Naringin: A Systematic Review. Image Credit: New Africa / Shutterstock
Systematic Review: Endothelial and Cardiovascular Effects of Naringin: A Systematic Review. Image Credit: New Africa / Shutterstock
In a recent study published in the journal Nutrients, researchers evaluated the cardiovascular and endothelial effects of naringin, a flavonoid found in citrus fruits.
Cardiovascular diseases are the leading cause of death worldwide. Among the various cardioprotective dietary bioactive compounds, flavonoids have gained significant attention for their antioxidant and anti-inflammatory capacities. Naringin is a flavanone glycoside mainly found in citrus fruits, especially in mandarin oranges and grapefruit. It has attracted considerable interest due to its multifaceted biological actions and potential cardioprotective role, though its clinical translation is limited by low oral bioavailability (<5%), prompting research into advanced delivery systems like liposomal encapsulation.
About the Study
In the present systematic review, researchers evaluated the cardiovascular and endothelial effects of naringin across cellular, animal, and human studies. First, they searched the Web of Science, PubMed, Embase, and Scopus databases to identify relevant articles published from January 2000 to June 2025. Original experimental research studies evaluating the effects of naringin on myocardial or endothelial function were included.
Reviews, editorials, abstracts, and studies without cardiovascular endpoints were excluded. Titles/abstracts were screened, followed by full text analysis based on the Population, Intervention, Comparator, and Outcomes (PICO) framework. Studies in human subjects, cell cultures, and animal models were retained. Cardiovascular or endothelial function outcomes included myocardial infarct size, blood pressure, markers of endothelial function, and cardiac remodeling, among others.
Data on study type, dose, model, treatment duration, endpoints, and mechanistic findings were extracted. A narrative synthesis approach was used due to heterogeneity in model systems, study design, and endpoints. A qualitative synthesis was performed to stratify results by primary endpoints (myocardial or endothelial function) and model type (human, cell, animal).
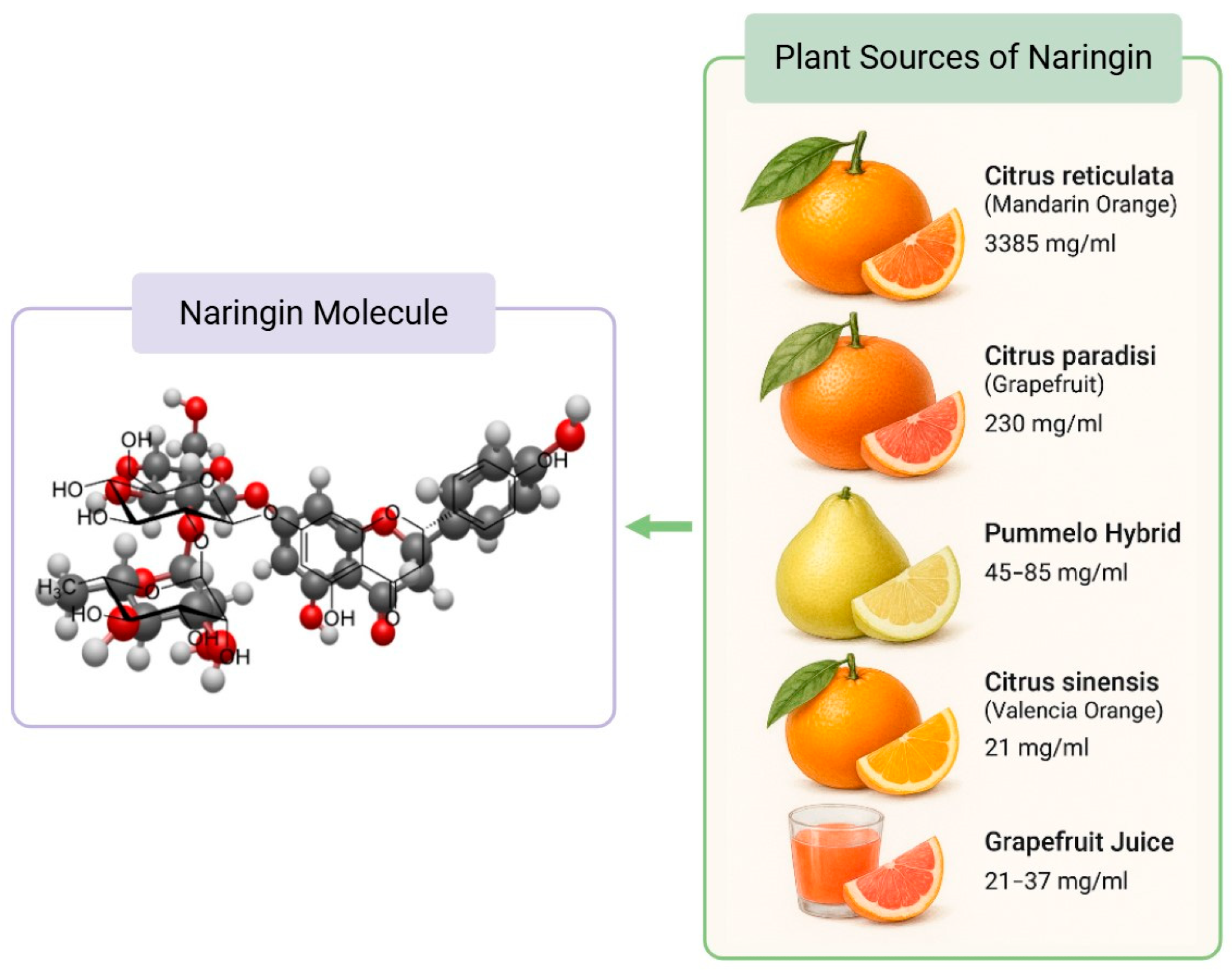
Naringin Molecular Structure and Citrus Food Sources. The concentration of naringin in plant sources were obtained from Alam et al.
Findings
The database search identified 2,884 unique records. The full texts of 165 records were assessed for eligibility, and 62 studies were included. These included 28 in vitro, 29 animal, and five human studies. Eight in vitro studies focused on endothelial cells and showed that naringin had protective effects on vascular endothelial cells via suppression of NF-κB signaling and adhesion molecules (e.g., VCAM-1, ICAM-1). Naringin attenuated inflammation activation and preserved normal function in cultured human endothelial cells.
Nineteen in vitro studies were on cardiovascular cell types, including five on vascular smooth muscle cells (VSMCs), and 14 on cardiac cells. Naringin was found to blunt apoptotic and hypertrophic responses in cardiomyocyte and cardiomyoblast models through modulation of PI3K/Akt and Nrf2 pathways. The anti-hypertrophic effect was related to its ability to inhibit downstream ion transporters and carbonic anhydrase II. Moreover, naringin has been shown to protect cardiomyocytes from simulated in vitro ischemia-reperfusion (I/R) by inhibiting ferroptosis and cGAS-STING pathways.
In a model of hypoxia/reoxygenation injury, naringin reduced oxidative stress, improved cell survival, and preserved mitochondrial membrane potential post-injury. Naringin has been shown to protect cardiomyocytes against doxorubicin-induced cardiotoxicity by reducing reactive oxygen species (ROS) generation and apoptosis. Further, naringin has been found to exert anti-atherogenic effects in VSMCs by curbing abnormal migration and proliferation.
Among animal studies, 15 used metabolic disorder models, with nine specifically focusing on myocardial I/R injury or hypertension. Animal models of endothelial injury and hyperlipidemia have demonstrated the anti-atherosclerotic effects of naringin. In rabbit models fed cholesterol, chronic naringin treatment reduced atherosclerotic lesion development.
Studies on hypercholesterolemic rabbits reported significant attenuation of aortic atherosclerosis with naringin treatment, associated with reduced expression of intercellular adhesion molecule 1 (ICAM-1) in the endothelium. In an atherosclerosis-prone mouse model, naringin inhibited plaque formation, protected vascular endothelium, and promoted endothelial nitric oxide synthase (eNOS) protein expression via PI3K/Akt activation.
Moreover, naringin has demonstrated anti-hypertensive effects linked to renin-angiotensin system (RAS) modulation, preventing cardiac remodeling. Studies on animal models of diet-induced metabolic syndrome have reported that naringin reduces cardiac hypertrophy and apoptosis. Beyond ischemia-reperfusion, benefits extended to diabetic cardiomyopathy, sepsis-induced myocardial dysfunction, and doxorubicin cardiotoxicity models.
In addition, naringin has consistently demonstrated cardioprotective effects in animal models of I/R injury and myocardial infarction (MI). For instance, naringin pretreatment significantly improved cardiac function and reduced myocardial damage in a rat model of I/R injury. This cardioprotection was associated with reductions in myocardial oxidative stress, inflammation, and apoptosis via PI3K/Akt and Nrf2/GPX4 pathways. In a rat model of MI, naringin pretreatment prevented myocardial necrosis and oxidative stress.
Naringin was found to improve cardiac function and histology in diabetic cardiomyopathy models and attenuate sepsis and lipopolysaccharide-induced myocardial dysfunction in other models. Further, compared to preclinical studies, few studies have examined the effects of naringin in humans. Although still limited, evidence on the cardiovascular effects of naringin in humans stems from dietary intervention studies and clinical trials.
A randomized controlled trial reported significant improvements in cardiometabolic parameters in adults who received naringin for 90 days, showing a favorable lipid-modulating effect. Notably, one trial also documented improved arterial stiffness (reduced pulse wave velocity) with naringin-rich grapefruit juice. A dietary intervention study of adults with moderate hypercholesterolemia reported that naringin intake for eight weeks did not change plasma cholesterol levels; this lack of effect might be due to insufficient dose or treatment duration, as effective preclinical doses translate to ~1 g/day in humans.
Conclusions
In sum, a substantial body of evidence positions naringin as a potent compound with cardiovascular benefits. Preclinical studies have documented its ability to protect the myocardium and improve endothelial function through multi-targeted actions on oxidative stress (Nrf2), inflammation (NF-κB), cell survival (PI3K/Akt), and RAS modulation. It can suppress oxidative stress and inflammation, preserve endothelial integrity, and activate pro-survival signaling in cells.
Despite the positive findings of naringin, further research is needed to define optimal dosing, improve bioavailability, and validate effects in large-scale human trials to cement its role in clinical practice.