A new review highlights how diabetes and weight loss drug tirzepatide could disrupt Alzheimer's disease by repairing brain metabolism and reducing inflammation.
 Study: Tirzepatide: a novel therapeutic approach for Alzheimer’s disease. Image credit: Atthapon Raksthaput/Shutterstock.com
Study: Tirzepatide: a novel therapeutic approach for Alzheimer’s disease. Image credit: Atthapon Raksthaput/Shutterstock.com
In a recent review published in the Metabolic Brain Disease, a group of authors explored the potential molecular mechanisms through which Tirzepatide (TRZ) exerts neuroprotective effects in Alzheimer’s disease (AD).
Background
AD affects over 55 million people globally and remains the leading cause of progressive loss of memory and cognitive abilities, commonly referred to as dementia. It is linked to numerous metabolic disorders like Type 2 diabetes (T2D) and obesity, causing low-grade chronic inflammation and oxidative stress, which can further lead to neurodegeneration.
Growing evidence shows that insulin resistance (IR) in the brain can challenge neural function, which is why AD is often termed “Type 3 diabetes.” Current drugs like TRZ, which activates both glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP) receptors, are under investigation for their neuroprotective potential. Further research is needed to validate its neuroprotective potential in clinical settings.
Understanding Alzheimer’s Disease Pathophysiology
AD is primarily driven by two pathological features: extracellular amyloid beta (Aβ) plaque accumulation and intracellular neurofibrillary tangles composed of tau protein. These anomalies disrupt neuronal communication, promote inflammation, and trigger cell death.
While rare inherited AD cases are caused by mutations in the amyloid precursor protein (APP) and presenilin genes, most occur sporadically. They are linked to factors like IR and long-term inflammation. The failure to adequately clear Aβ from the brain and disruptions in insulin signaling are central to disease progression.
Tirzepatide’s Mechanism of Action
TRZ is approved for managing T2D and obesity. It not only improves blood sugar levels but also crosses the blood-brain barrier, making it a promising treatment for neurogenerative diseases. In peripheral systems, it reduces body weight, improves lipid profiles, and lowers inflammatory markers. These actions indirectly benefit the brain by minimizing the metabolic dysfunctions contributing to AD.
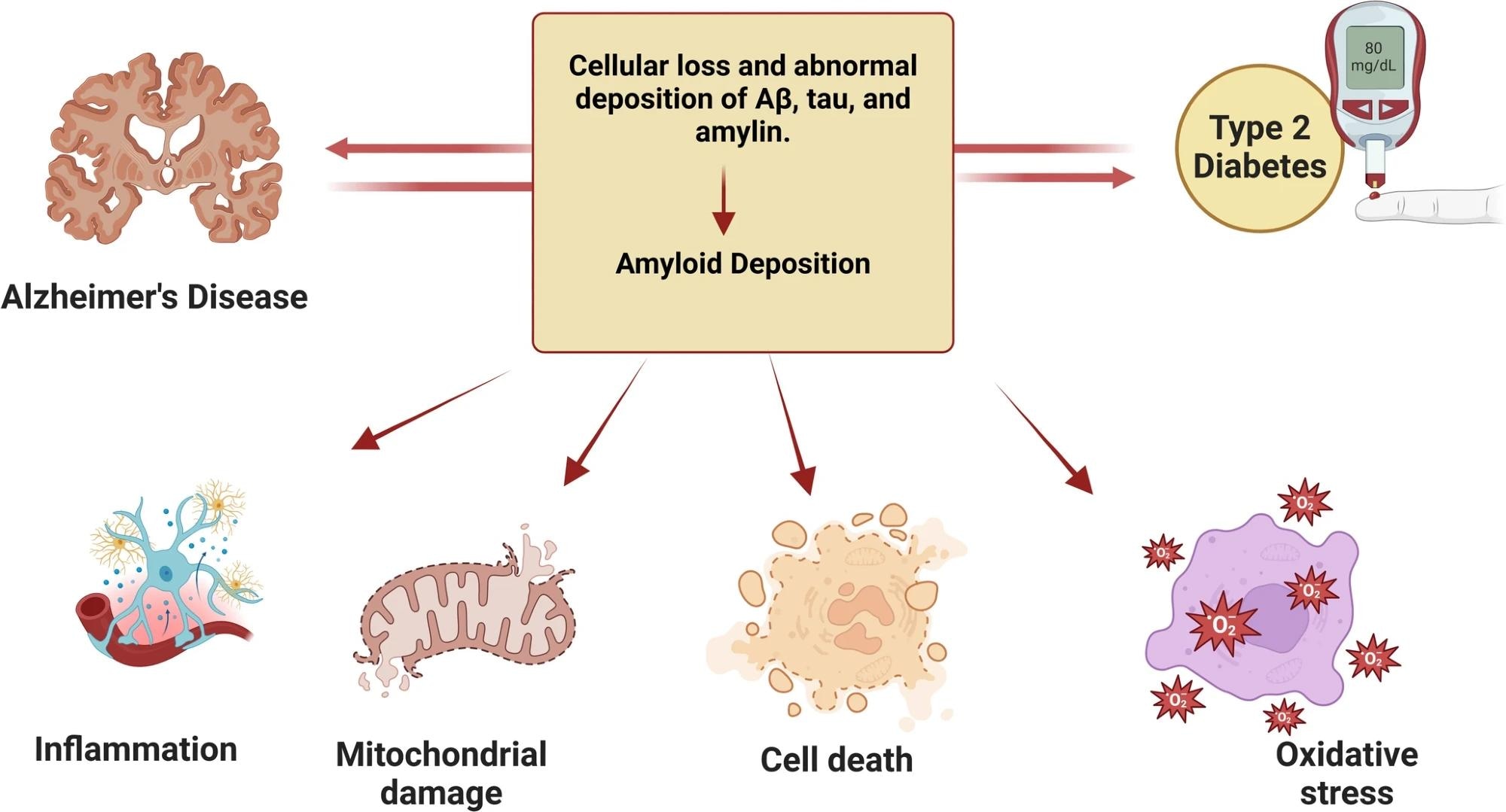 Mechanism of action of TRZ
Mechanism of action of TRZ
Neuroinflammation and Insulin Resistance
AD is increasingly recognized as being associated with brain IR. In this condition, insulin signaling within the central nervous system (CNS) is impaired, leading to synaptic dysfunction and cognitive deficits.
TRZ improves brain insulin sensitivity through the phosphoinositide 3-kinase/protein kinase B/glycogen synthase kinase three beta (PI3K/AKT/GSK3β) signaling pathway. Preclinical studies have shown that this pathway restores glucose metabolism in neurons, reduces Aβ deposition, and preserves cognitive function.
Beyond improving insulin signaling, TRZ also helps calm brain inflammation by reducing activity in key inflammatory pathways like NLRP3 inflammasome and nuclear factor kappa-B (NF-Κb), which are known to drive microglial activation and neuroinflammation in AD.
Mice treated with TRZ showed reduced levels of these markers, indicating that the drug can suppress neuroinflammation and potentially slow disease progression. However, the review notes that not all preclinical results have been uniformly positive. One study found that TRZ and the related drug semaglutide did not reduce amyloid plaque or improve cognitive deficits in specific transgenic mouse models of AD.
The authors suggest that differences in experimental design, dosing, or the specific animal models used may account for these mixed results. However, this highlights the need for further research before drawing firm conclusions.
Effects on Obesity-Related Brain Dysfunction
Obesity raises the risk of AD by causing leptin resistance, disrupting satiety and cognitive function, and chronic inflammation, making the hormone less effective at controlling appetite and brain function. TRZ alters leptin sensitivity by upregulating adiponectin and enhancing the leptin-adiponectin axis, improving hippocampal function.
It also induces weight loss, indirectly reducing inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), which are implicated in neurodegenerative changes.
Preclinical models demonstrate that TRZ-treated obese mice had improved memory performance and lower brain levels of amyloid plaques. The drug also normalized the levels of glucose transporters like glucose transporter type 1 (GLUT1), glucose transporter type 3 (GLUT3), and glucose transporter type 4 (GLUT4), which are critical for neuronal energy supply. These outcomes highlight TRZ’s multifaceted action in addressing the metabolic roots of cognitive decline.
Tirzepatide and Autophagy
Autophagy, the brain’s mechanism for clearing damaged proteins and organelles, is significantly impaired in AD. TRZ activates autophagy-related genes and enzymes, promoting the clearance of Aβ and improving neuronal health. It achieves this via the PI3K/AKT pathway, enhancing cellular resilience to stress and delaying neuronal aging. This is particularly relevant in individuals with T2D, where defective autophagy is a shared feature with AD.
Neurogenesis and Synaptic Health
TRZ supports neuronal regeneration and boosts the production of brain-derived neurotrophic factor (BDNF) and cyclic adenosine monophosphate response element-binding protein (CREB), both essential for memory and learning. It positively regulates microRNAs (miRNAs), such as miR-212-3p and miR-29c-5p, which control apoptotic pathways and APP processing.
Additionally, TRZ therapy upregulates proteins involved in synaptic plasticity, such as growth-associated protein 43 (GAP-43) and microtubule-associated protein 2 (MAP2).
These molecular changes suggest that TRZ prevents neurodegeneration and supports brain repair. While most of these findings come from animal models, they offer compelling insights into the drug’s potential as a disease-modifying therapy for AD.
The review emphasizes that the exact mechanisms behind TRZ’s neuroprotective effects are still not fully understood, and these findings have not yet been applied to human patients.
Conclusions
TRZ offers a promising therapeutic avenue for AD by targeting both peripheral and central mechanisms. It reduces systemic inflammation, corrects brain IR, promotes autophagy, and enhances synaptic health. These multifactorial effects address the metabolic and neurodegenerative components of the disease simultaneously.
Although current evidence stems largely from preclinical models, TRZ’s impact on key disease pathways warrants further investigation in clinical trials. Notably, the review cautions that some animal studies have shown no benefit, so the clinical potential of TRZ for AD is still uncertain. If validated, it could represent a transformative strategy in managing AD alongside T2DM and obesity.
Download your PDF copy now!
Journal reference:
-
Alshehri, G.H., Al-Kuraishy, H.M., Waheed, H.J., Al-Gareeb, A.I., Faheem, S.A., Alexiou, A., Papadakis, M. and El-Saber Batiha, G., (2025). Tirzepatide: a novel therapeutic approach for Alzheimer’s disease. Metabolic Brain Disease, 40(5), pp.1-13. Doi- https://doi.org/10.1007/s11011-025-01649-z. https://link.springer.com/article/10.1007/s11011-025-01649-z