Introduction
What are aluminum adjuvants?
Mechanisms of action
Safety and pharmacokinetics
Myths and misconceptions
Special considerations
Conclusions
References
Further reading
This article reviews the science behind aluminum adjuvants in vaccines, examining their mechanisms, safety, and the evidence that dispels common myths. It also discusses recent research and innovations that shape the future of vaccine adjuvant technology.
 Image Credit: IM Imagery / Shutterstock.com
Image Credit: IM Imagery / Shutterstock.com
Introduction
Adjuvants are substances added to vaccines to enhance their efficacy, quality, safety, and/or durability in vivo. Aluminum salts, which are one of the most widely used human vaccine adjuvants, have facilitated the development of highly effective vaccines from purified or inactivated components that would otherwise be poorly immunogenic. Adjuvants may also be used to reduce antigen quantities and the number of immunizations required for protection.2,3
Despite being critical in distributing billions of vaccine doses worldwide and potentially saving millions of lives, aluminum-containing vaccines are increasingly subject to public apprehension and vaccine hesitancy. Often fueled and exacerbated by misinformation, many of these claims posit unsubstantiated connections between aluminum adjuvants and a wide range of chronic disorders.4
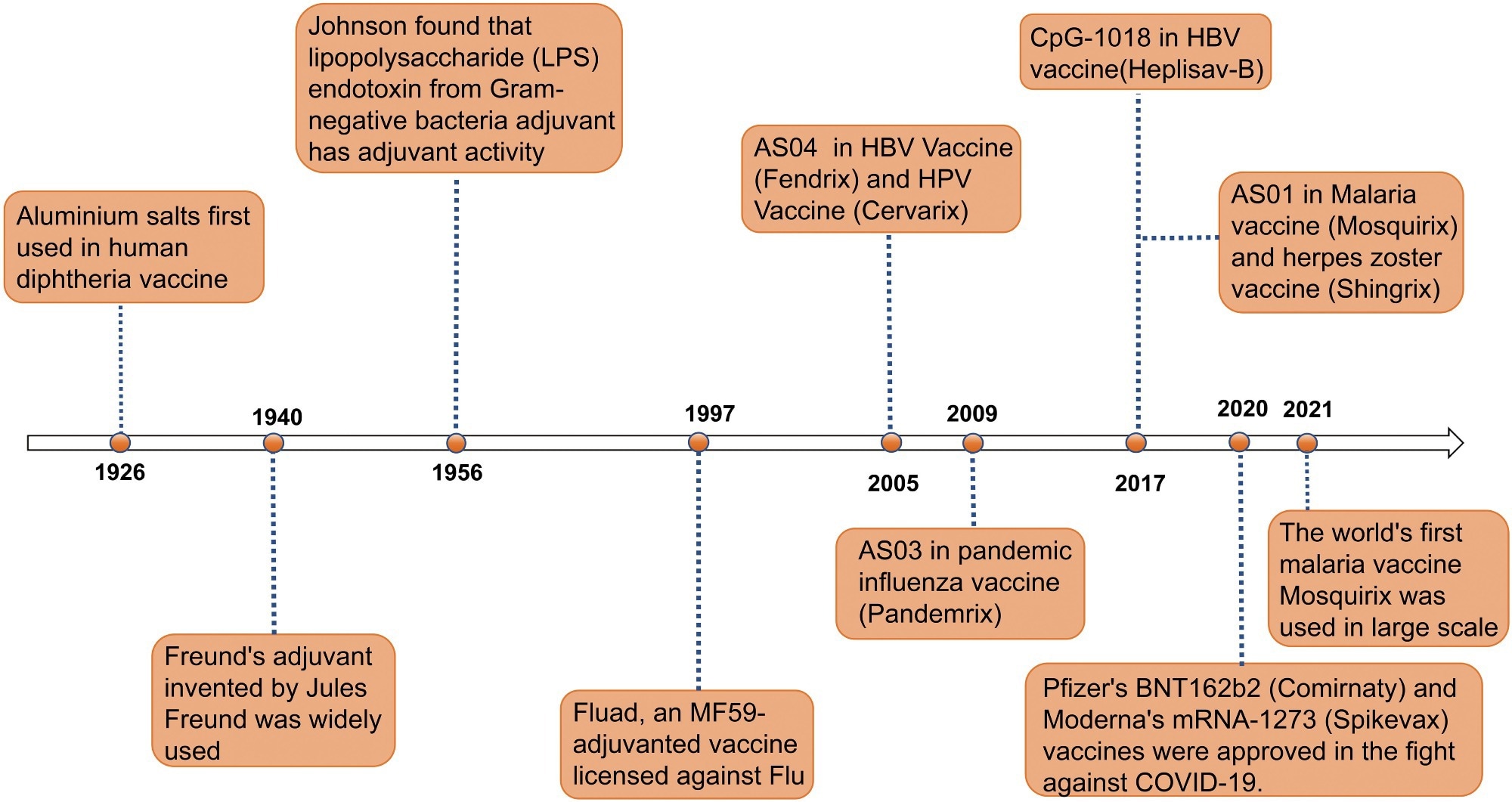 Timeline of major events in the research history of vaccine adjuvants. Since the first use of aluminum salt adjuvants in diphtheria vaccines in 1926, adjuvant technology has gradually evolved. In 1940, the invention of Freund’s adjuvant provided a new direction for enhancing immune responses in vaccines. In 1956, the discovery of the adjuvant activity of lipopolysaccharide (LPS) endotoxins further expanded the range of available adjuvants. In 1997, the application of the MF59 adjuvant in the Fluad influenza vaccine marked the significant role of adjuvants in influenza prevention and treatment. In 2005, the AS04 adjuvant was first used in HBV and HPV vaccines (Fendrix and Cervarix). In 2009, the AS03 adjuvant was used in the pandemic influenza vaccine Pandemrix. In 2017, the CpG-1018 and AS01 adjuvants were applied to the HBV vaccine (Heplisav-B) and malaria and shingles vaccines (Mosquirix and Shingrix), respectively. In 2020, Pfizer’s BNT162b2 vaccine (Comirnaty) and Moderna’s mRNA-1273 vaccine (Spikevax) were approved, making a significant contribution to the fight against COVID-19. In 2021, the world’s first malaria vaccine RTS,S/AS01 began large-scale use, further proving the role of adjuvants in enhancing vaccine efficacy. This timeline illustrates the continuous innovation and breakthroughs of adjuvants in the field of vaccines.2
Timeline of major events in the research history of vaccine adjuvants. Since the first use of aluminum salt adjuvants in diphtheria vaccines in 1926, adjuvant technology has gradually evolved. In 1940, the invention of Freund’s adjuvant provided a new direction for enhancing immune responses in vaccines. In 1956, the discovery of the adjuvant activity of lipopolysaccharide (LPS) endotoxins further expanded the range of available adjuvants. In 1997, the application of the MF59 adjuvant in the Fluad influenza vaccine marked the significant role of adjuvants in influenza prevention and treatment. In 2005, the AS04 adjuvant was first used in HBV and HPV vaccines (Fendrix and Cervarix). In 2009, the AS03 adjuvant was used in the pandemic influenza vaccine Pandemrix. In 2017, the CpG-1018 and AS01 adjuvants were applied to the HBV vaccine (Heplisav-B) and malaria and shingles vaccines (Mosquirix and Shingrix), respectively. In 2020, Pfizer’s BNT162b2 vaccine (Comirnaty) and Moderna’s mRNA-1273 vaccine (Spikevax) were approved, making a significant contribution to the fight against COVID-19. In 2021, the world’s first malaria vaccine RTS,S/AS01 began large-scale use, further proving the role of adjuvants in enhancing vaccine efficacy. This timeline illustrates the continuous innovation and breakthroughs of adjuvants in the field of vaccines.2
What are aluminum adjuvants?
Aluminum adjuvants are often referred to as "alum," but this term specifically applies to potassium aluminum sulfate, which was used historically. Most modern vaccines use aluminum hydroxide (Al(OH)3) or aluminum phosphate (AlPO4), which differ in their chemistry, charge, and persistence in tissues. Potassium aluminum sulfate [KAl(SO4)2] is rarely used now.1-4
Initially used in the 1930s to enhance diphtheria and tetanus toxoids, aluminum adjuvants are widely incorporated into vaccines for diphtheria, tetanus, and acellular pertussis (DTaP), Haemophilus influenzae type b (Hib), hepatitis A, hepatitis B, human papillomavirus (HPV), and pneumococcus vaccines.4,5
Several global health authorities, including the World Health Organization (WHO) and the United States Food and Drug Administration (FDA), recognize the safety and importance of aluminum adjuvants. These agencies have also established science-based frameworks for the approval and quality assessment of vaccines that contain these additives.5,6
Aluminum adjuvants are highly effective at promoting antibody (Th2-type) responses, but have limited ability to stimulate strong T cell (Th1 or cytotoxic) responses. For this reason, newer adjuvants, such as oil-in-water emulsions (MF59, AS03), TLR agonists, and combination adjuvant systems, are used in some modern vaccines, particularly where robust cellular immunity is required.2
What ingredients are in vaccines? | The Vaccines Project, Episode 2
Mechanisms of action
The ‘depot effect' theory suggests that aluminum salts form a repository at the injection or target site, which leads to the slow release of their antigen. Aluminum salts may also create a localized concentration of antigens while facilitating the accumulation of immune cells in the area. However, current research indicates that the immune-stimulating effect of aluminum is not due solely to antigen persistence at the injection site ("depot effect"), but also involves direct recruitment and activation of antigen-presenting cells and induction of the NLRP3 inflammasome. Several studies have reported that aluminum can be cleared from the injection site more rapidly than the depot model would suggest.1,2
Most researchers agree that aluminum adjuvants act as potent activators of the innate immune system by inducing a cascade of reactions. For example, aluminum-adjuvanted vaccines create a localized inflammatory environment that provides a recruitment signal for key antigen-presenting cells (APCs), such as dendritic cells and macrophages, to the target site.1
Following APC phagocytosis, aluminum particles induce lysosomal stress, which subsequently activates assembly of the NLRP3 inflammasome. Thereafter, the activated NLRP3 inflammasome initiates a signaling cascade that involves cleavage and secretion of potent pro-inflammatory cytokines, including interleukin-1β (IL-1β) and IL-18.1,2
Aluminum adjuvants are particularly effective at driving a T helper 2 (Th2)-type immune response, which is characterized by the robust activation of B-cells and antibody production. As a result, aluminum adjuvants are particularly attractive in manufacturing vaccines that protect against extracellular bacteria and toxins. They are less effective at inducing Th1 or cytotoxic T cell responses, which limits their use in vaccines against some intracellular pathogens.1, 2,
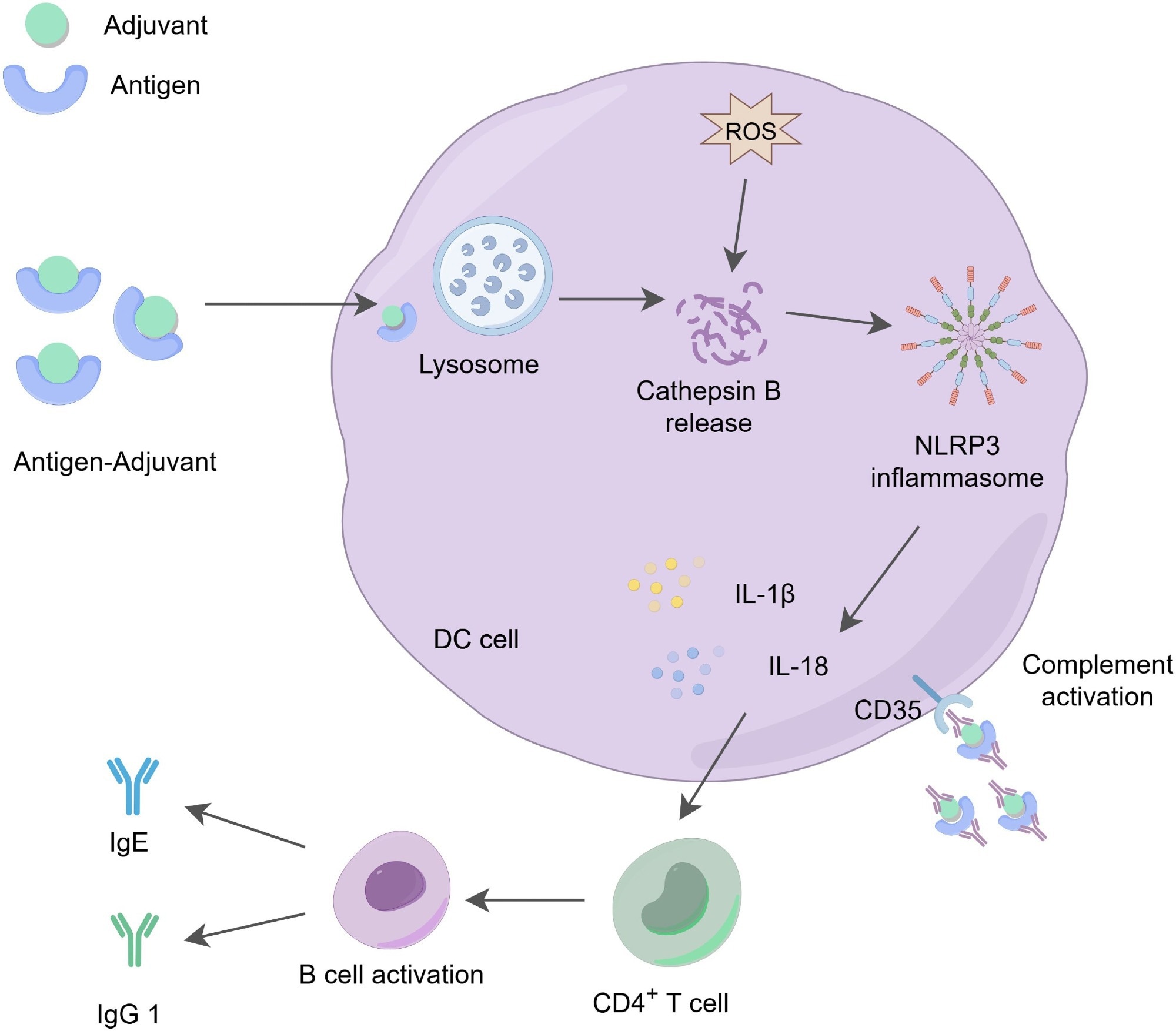 The action mechanism of aluminum adjuvants. Aluminum adjuvants form microparticles by adsorbing soluble antigens, which promotes the phagocytosis of these antigens by APCs. The phagocytosed aluminum-adjuvant-antigen complexes indirectly promote the production of reactive oxygen species (ROS), activate the release of cathepsin B in the lysosome, triggering the activation of the NLRP3 inflammasome and stimulating the production of IL-1β and IL-18, which play a role in regulating immune responses. At the same time, aluminum adjuvants stimulate the activation and differentiation of CD4+ T cells, increasing the levels of IgG1 and IgE. These cytokines and immune factors are essential for effective antibody-mediated immune protection. Additionally, dendritic cells can recruit and deposit antigen-adjuvant-antibody complexes through the CD35 receptor, further enhancing receptor signaling on both B cells and dendritic cells, thereby promoting immune effector functions.2
The action mechanism of aluminum adjuvants. Aluminum adjuvants form microparticles by adsorbing soluble antigens, which promotes the phagocytosis of these antigens by APCs. The phagocytosed aluminum-adjuvant-antigen complexes indirectly promote the production of reactive oxygen species (ROS), activate the release of cathepsin B in the lysosome, triggering the activation of the NLRP3 inflammasome and stimulating the production of IL-1β and IL-18, which play a role in regulating immune responses. At the same time, aluminum adjuvants stimulate the activation and differentiation of CD4+ T cells, increasing the levels of IgG1 and IgE. These cytokines and immune factors are essential for effective antibody-mediated immune protection. Additionally, dendritic cells can recruit and deposit antigen-adjuvant-antibody complexes through the CD35 receptor, further enhancing receptor signaling on both B cells and dendritic cells, thereby promoting immune effector functions.2
Safety and pharmacokinetics
Aluminum salt-containing vaccines are usually injected intramuscularly, following which the salts slowly dissolve and are absorbed into the bloodstream. Thereafter, aluminum salts bind to the transporter protein transferrin until being cleared by the kidneys and excreted in urine.3,7
In individuals with normal kidney function, the biological half-life of aluminum in the blood is less than 24 hours. Of the trace aluminum that remains, the largest amount is deposited in the bone, with smaller amounts potentially present in other organs, including the brain. Aluminum phosphate adjuvants are cleared faster from tissues than aluminum hydroxide. There is no evidence that the trace amounts of aluminum remaining after vaccination are harmful at the doses used in vaccines.3,6,7
Public concern often focuses on the quantity of aluminum in vaccines; however, these doses are minimal as compared to daily dietary intake. For example, infants, who are likely the most vulnerable demographic for potential toxicity, consume about 117 mg of aluminum from baby formula by their sixth month of life. This intake of aluminum is significantly greater than the cumulative 4.4 mg dose that infants will be exposed to after completing their entire Centers for Disease Control and Prevention (CDC)-recommended vaccination schedule.3
Pharmacokinetic modeling by the FDA and other research institutions confirms that, even with the complete childhood vaccination schedule, this amount of aluminum exposure is significantly less than the minimal risk level (MRL) set by the Agency for Toxic Substances and Disease Registry (ATSDR).6,7
Even when continuously monitoring blood aluminum levels in infants immediately following vaccine administration, previous clinical studies could not detect a rise in blood aluminum levels. Thus, the vaccine dose of aluminum is insignificant relative to the continuous exposure from food and water ingestion.3
Local reactions, such as mild redness, swelling, granuloma formation, or persistent small nodules at the injection site, may occur with aluminum-adjuvanted vaccines, but these are generally non-serious and self-limiting.1
Myths and misconceptions
Despite scientific evidence overwhelmingly supporting the safety of aluminum as an adjuvant, public anxiety against its use continues to rise. Many of these individuals incorrectly believe that aluminum adjuvants increase the risk of various chronic diseases, including autism spectrum disorder (ASD), neurotoxicity, and autoimmune conditions.4
These misconceptions have been traced to previous exploratory ecological studies correlating the number of vaccines with ASD prevalence and/or animal studies using high and non-physiological doses of aluminum.4,1 Importantly, these study designs are prone to confounding and cannot establish causation.
In contrast, the highest level of evidence from extensive, longitudinal human cohort studies consistently fails to support these claims. For example, a 2025 Danish nationwide cohort study monitoring over 1.2 million children for up to 24 years concluded that there was no evidence to support an increased risk for any of the 50 chronic disorders assessed in association with early childhood exposure to aluminum-adjuvanted vaccines. Other large-scale studies have similarly found no association between recommended vaccine use and increased risk for autism, developmental delays, or autoimmune conditions.4,10
 Image Credit: Gorodenkoff / Shutterstock.com
Image Credit: Gorodenkoff / Shutterstock.com
Special considerations
Although the overall safety profile of aluminum adjuvants is well-established, some patients may be at a greater risk of experiencing adverse effects. For example, a 2022 observational study reported a statistically significant association between cumulative vaccine-associated aluminum exposure before the age of two and the subsequent development of persistent asthma.8
This single study found a positive association between cumulative aluminum exposure from vaccines and persistent asthma (adjusted hazard ratios of 1.26 and 1.19 per 1 mg increase, depending on eczema status), but the effect size was small and the authors emphasized the possibility of residual confounding and lack of causality. Additional investigation of this hypothesis is warranted, but current evidence overwhelmingly supports the safety of the vaccine schedule.8 These findings emphasize the importance of continuous post-licensure safety monitoring to investigate even rare outcomes.
Infants with renal function impairments and immunocompromised individuals are other groups closely monitored for potential complications.7,10
Conclusions
Aluminum adjuvants remain an essential, effective, and well-studied component of modern vaccines. Despite widespread misinformation, almost a century of scientific evidence, culminating in large-scale epidemiological studies involving millions of children, confirms their exceptional safety profile.1,2,10
To address public concerns, mitigate the potential for adverse events in high-risk populations, and overcome the limitations of aluminum salts as adjuvants, researchers are currently developing novel adjuvants that can be tailored to induce specific types of immunity. These next-generation adjuvants, particularly combination approaches and nanoformulations incorporating Toll-like receptor (TLR) agonists, represent the future of vaccinology. Examples of licensed non-aluminum adjuvants include MF59 (an oil-in-water emulsion), AS01 (liposome-based with TLR4 agonist), AS04 (aluminum plus monophosphoryl lipid A), and CpG 1018 oligonucleotides, used in certain influenza, shingles, and hepatitis B vaccines.2,10
References
- HogenEsch, H. (2013). Mechanism of Immunopotentiation and Safety of Aluminum Adjuvants. Frontiers in Immunology 3. DOI:10.3389/fimmu.2012.00406, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2012.00406/full
- Xing, J., Zhao, X., Li, X., Fang, R., Sun, M., Zhang, Y., & Song, N. (2025). The recent advances in vaccine adjuvants. Frontiers in Immunology, 16. DOI:10.3389/fimmu.2025.1557415, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1557415/full
- Children's Hospital of Philadelphia. (2022). Aluminum in vaccines. Vaccine Education Center. https://www.chop.edu/vaccine-education-center/vaccine-safety/vaccine-ingredients/aluminum. Accessed on 22 July 2025.
- Andersson, N. W., Bech Svalgaard, I., Hoffmann, S. S., & Hviid, A. (2025). Aluminum-Adsorbed Vaccines and Chronic Diseases in Childhood. Annals of Internal Medicine. DOI:10.7326/annals-25-00997, https://www.acpjournals.org/doi/10.7326/ANNALS-25-00997
- U.S. Food and Drug Administration. (2024). Common ingredients in U.S. licensed vaccines. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/common-ingredients-fda-approved-vaccines. Accessed on 22 July 2025.
- World Health Organization. (2012). Global Advisory Committee on Vaccine Safety statement on safety of aluminium adjuvants. https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/topics/adjuvants. Accessed on 22 July 2025.
- Mitkus, R. J., King, D. B., Hess, M. A., Koutsouyanni, K., & Wall, S. (2011). Updated aluminum pharmacokinetics following infant exposures through diet and vaccination. Vaccine, 29(51), 9538–9543. DOI:10.1016/j.vaccine.2011.09.124, https://www.sciencedirect.com/science/article/abs/pii/S0264410X11015799
- Daley, M. F., Reifler, L. M., Glanz, J. M., Hambidge, S. J., Getahun, D., Irving, S. A., Nordin, J. D., McClure, D. L., Klein, N. P., Jackson, M. L., Kamidani, S., Duffy, J., & DeStefano, F. (2023). Association Between Aluminum Exposure From Vaccines Before Age 24 Months and Persistent Asthma at Age 24 to 59 Months. Academic Pediatrics, 23(1), 37–46. DOI:10.1016/j.acap.2022.08.006, https://www.sciencedirect.com/science/article/pii/S187628592200417X
- Centers for Disease Control and Prevention. (2023). Adjuvants and vaccines. https://www.cdc.gov/vaccine-safety/about/adjuvants.html. Accessed on 22 July 2025.
- Hayek, H., Hasan, L., Amarin, J. Z., Qwaider, Y. Z., Hamdan, O., Rezende, W., Dee, K. C., Chappell, J. D., & Halasa, N. B. (2025). Vaccine Adjuvants in the Immunocompromised Host: Science, Safety, and Efficacy. Transplant Infectious Disease, e70053. DOI:10.1111/tid.70053, https://onlinelibrary.wiley.com/doi/10.1111/tid.70053
Further Reading
Last Updated: Aug 3, 2025