Jan 6 2016
Evidence for carcinogenic chromium(VI) compounds in chromium(III)-treated living cells
Chromium supplements are widely consumed for their antidiabetic activity as chromium(III) enhances the insulin sensitivity of cells. In particular, orthomolecular practitioners believe in the beneficial effects of providing the body with extra amounts of essential trace elements. As Australian and American scientists now report in the journal Angewandte Chemie, chromium(III) dietary supplements are oxidized to a certain extent in living cells to their carcinogenic and genotoxic chromium(V) and chromium(VI) counterparts, which raises questions about potential risks of such therapies.
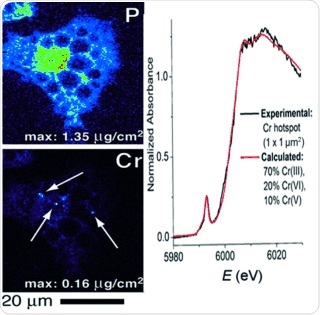
In the body, chromium(III) is known to be able to enhance the effects of insulin and oral antidiabetic drugs. Thus it has been proposed that the daily intake of a certain amount of chromium lowers the blood glucose level. However, in higher oxidation states, chromium compounds can have detrimental effects on DNA and thus can cause cancer. There is growing concern about possible oxidation pathways during the metabolic transformation of the chromium(III)-containing drugs in the cell. With the aim at getting more insight into the fate of the chromium compounds after their intake, Peter A. Lay from the University of Sydney, Australia, and his colleagues employed X-ray fluorescence microscopy (XFM) elemental mapping and microfocus X-ray absorption near-edge structure (µ-XANES) analysis on single chromium(III)-treated adipocytes, making use of the Australian Synchrotron facility and of resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility.
By carrying out XFM analysis, Lay and his colleagues found that the chromium was confined in "hotspots", and the µ-XANES data revealed that these hotspots did not only contain chromium(III). The authors conclude: "This finding unambiguously confirmed the presence of high oxidation states of chromium." After having established the presence of higher oxidation states, the scientists also modelled the possible chromium compounds and identified chromium(V) and chromium(VI) compounds.
The question remained why the chromium was oxidized in the overall reducing environment of the cells. The authors present a possible and plausible explanation: During cell signaling, and especially insulin signaling, strong oxidants such as hydrogen peroxide are formed, which will trigger the formation of reactive chromium(V) and chromium(VI) intermediates. "This raises concern over the possible carcinogenicity of chromium(III) compounds and the risks of long-term chromium(III) nutritional supplementation," the authors say. Although concerns have been raised for some time about the efficacy and safety of such supplements, many authorities and a large community of orthomolecular practitioners still recommend such treatments. Thus Lay and his colleagues urge for future studies: "In light of these findings, there is a need for epidemiological studies to ascertain whether chromium(III) supplements alter cancer risk".