Jan 18 2018
Study of clinical trial professionals involved in RTSM/IRT reveals poor integration with other clinical platforms, slowness to build, and inability to support study changes as top issues
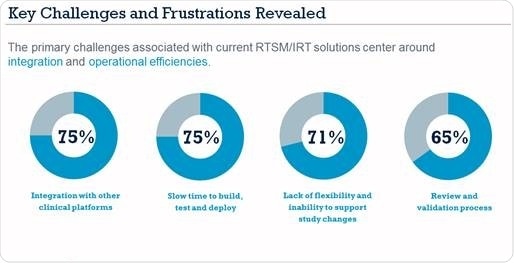
Oracle Health Sciences today announced the results of a new study, conducted in partnership with Informa Engage, which reveals the top challenges and frustrations for clinical trial professionals with current Randomization and Trial Supply Management/ Interactive Response Technology (RTSM/IRT) systems, as well as opportunities for improvement. Among the many findings, the study revealed the top three challenges in RTSM/IRT for over 70 percent of respondents: integration with other platforms, slowness to build, test and deploy new trials, and lack of flexibility and inability to support study changes.
Clinical trials are known for being complex and lengthy, and trial set up can account for a large portion of the time. Exploring the reasons for delay, the study found that 92% of respondents reported having to make changes to their RTSM/IRT systems due to study changes, and on an average of two times per study. Given these results, it’s no wonder that over half of the respondents believe they could conduct more trials if they were able to leverage self-service capabilities to build new trials and make mid-study changes on their own.
In addition, the survey revealed that the most anticipated change in RTSM/IRT within the next five years is the integration of IRT into the eClinical platform, followed by accelerated trial builds, and completely self-service solutions, which reflects a desire for the top current challenges to be addressed in the next generation of RTSM/IRT platforms.
“When you’re developing software to help people with a specific business process, such as clinical research, it’s important to stay in tune with the market to ensure you’re always innovating to address their top challenges and delighting them with a solution that makes their job easier,” said Steve Rosenberg, general manager, Oracle Health Sciences. “The findings from this research confirm the pain points that we address with our new Clinical One Randomization and Supplies Management Cloud Service and that we are aligned with what the market wants in a modern RTSM/IRT solution.”
Oracle recently announced https://www.oracle.com/corporate/pressrelease/clinical-one-061917.html">Oracle Clinical One, a cloud-based eClinical environment that is intended to redefine the way technology supports clinical research. Its first module, http://www.oracle.com/us/industries/health-sciences/randomization-supply-sales-sheet-3759059.pdf">Clinical One Randomization and Supplies Management, was designed with self-service in mind, enabling clinical teams to design, validate and deploy a study in days with the click of a button, effectively addressing the issues identified in this study.
The study, whose goal was to identify the top challenges facing users of RTSM/IRT solutions, highlights many of the issues important to clinical operations and trial management professionals. Data was gathered via an online survey completed by 254 professionals in clinical operations, trial management and related functional areas with direct involvement in both Clinical Trial Operations and RTMS/IRT solutions.