A groundbreaking phase 2a study reveals that rentosertib, an AI-developed TNIK inhibitor, could offer new hope for idiopathic pulmonary fibrosis patients after demonstrating improved lung function and a favorable safety profile.
 Study: A generative AI-discovered TNIK inhibitor for idiopathic pulmonary fibrosis: a randomized phase 2a trial. Image Credit: Pepermpron / Shutterstock
Study: A generative AI-discovered TNIK inhibitor for idiopathic pulmonary fibrosis: a randomized phase 2a trial. Image Credit: Pepermpron / Shutterstock
In a recent study in the journal Nature Medicine, researchers report the findings of rentosertib's (formerly ISM001-055) phase 2a clinical trial. Rentosertib is an AI-discovered TNIK inhibitor that shows promise in improving lung function, as measured by forced vital capacity, in patients with idiopathic pulmonary fibrosis (IPF). While still in clinical development, rentosertib represents a significant advancement as one of the few AI-discovered drugs to reach this stage of human trials and offers hope for a novel therapeutic approach for IPF. It is important to note that these early-phase results, while encouraging, do not guarantee future clinical benefit or regulatory approval.
The 12-week-long study investigated the safety and efficacy of several dosage combinations of rentosertib or an equivalent placebo. Study findings from 71 participants revealed that the overall rates of treatment-emergent adverse events (TEAEs) were generally similar across all study cohorts. However, treatment-related AEs were more common in the rentosertib groups compared to placebo, though serious treatment-related AEs were low and comparable. The 60 mg once-daily dose also demonstrated potential efficacy, increasing participants' forced vital capacity (FVC) by +98.4 ml, supporting rentosertib's inclusion in larger, longer-term studies.
Background
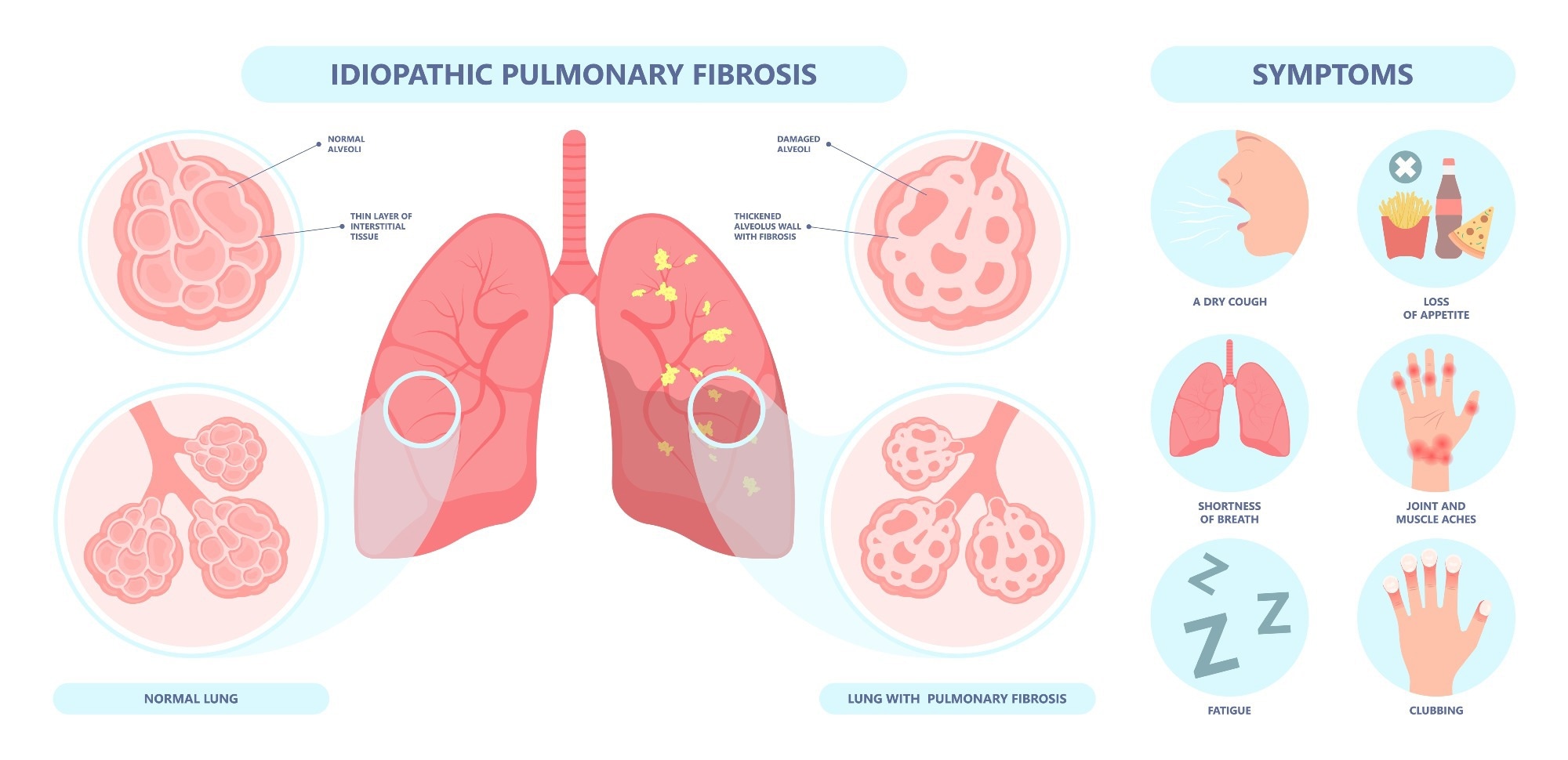
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, age-associated lung disease. It is characterized by the irreversible scarring and thickening of the lung tissue, resulting in death by respiratory failure.
While rare (10–60 cases per 100,000 Americans), the disease remains without a cure and inevitably results in patient death (median survival time = 2–4 yrs). Current pharmacological interventions (nintedanib and pirfenidone) are aimed at delaying disease progression, highlighting the need to discover more effective treatment agents. Unfortunately, conventional drug discovery is slow (10–15 yrs), tedious, and exceedingly expensive (US$2–3 billion).
Recent advances in generative artificial intelligence (AI) algorithms and research evaluating their performance have demonstrated that these tools can identify and design novel pharmaceuticals at a fraction of the time and economic costs of traditional approaches.
Previous research by the current study group leveraged generative AI to not only identify an IPF-associated drug target (Traf2- and Nck-interacting kinase [TNIK]) but also design an inhibitor molecule ('rentosertib,' formerly ISM001-055) with the potential to counteract TNIK's profibrotic and proinflammatory effects. Rentosertib has already cleared phase 0 and 1 clinical trials, demonstrating safety and high tolerance in healthy humans.
About the study
The present study reports the results of rentosertib's phase 2a clinical trials. The study employed a randomized, placebo-controlled, multi-dose trial design to investigate the safety and efficacy of rentosertib in a cohort of Chinese IPF patients between July 19, 2023, and June 11, 2024. The study recruited 128 IPF patients (age > 40 years), 57 of whom were excluded due to comorbidities or recent respiratory tract infections.
The remaining 71 patients were randomly assigned to one of four intervention cohorts: 30 mg of the drug once daily (QD, n = 18), 30 mg of the drug twice daily (BID, n = 18), 60 mg of the drug QD (n = 18), or placebo (n = 17). The study lasted 12 weeks and comprised frequent evaluations. The primary objective was to evaluate safety and tolerability, with the primary endpoint being the percentage of patients experiencing at least one treatment-emergent adverse event (TEAE). Other evaluations included forced vital capacity (FVC) metrics, questionnaire-assessed cough-associated quality of life (QOL), and blood samples collected at baseline (week 0), weeks 2, 4, 8, and 12 for pharmacokinetic (PK) assessments and biomarker analysis.
Endpoints were assessed using analysis of covariance (ANCOVA) analyses wherein treatment (drug or placebo) was coded as a fixed effect, and participants' baseline values were coded as covariates. PK parameters were determined from plasma concentrations, while proteomic profiling of serum samples was conducted to understand the mechanism of action and identify potential biomarkers of response.
Study findings
Of the 71 participants included in the clinical trial, 16 (placebo = 2, 30 mg rentosertib QD = 2, 30 mg rentosertib BID = 6, 60 mg rentosertib QD = 6) discontinued treatment prior to the study's completion due to adverse events (AEs) or unforeseen withdrawals. The most common AEs leading to discontinuation were related to liver injury or dysfunction (7 of 12 AE-related discontinuations), with diarrhea also being a factor. Notably, participants who discontinued were included in drug safety assessments.
Primary endpoint analyses revealed that the rates of overall treatment-emergent adverse events (TEAEs) were similar across all cohorts, with 70.6% of placebos, 72.2% of 30 mg QD, and 83.3% of both 30 mg BID and 60 mg QD groups reporting at least one TEAE. However, treatment-related AEs were more common among patients who received rentosertib (ranging from 50.0% to 77.8%) compared with placebo (29.4%). Serious treatment-related AEs were infrequent and comparable across treatment groups (0% in placebo, 5.6% in 30 mg QD, 11.1% in 30 mg BID, and 11.1% in 60 mg QD). The most common TEAEs included hypokalemia, abnormal hepatic function, diarrhea, and increased alanine aminotransferase (ALT). Four of the seven participants who withdrew due to liver toxicity were concurrently receiving nintedanib antifibrotic therapy.
Efficacy analyses revealed the potential therapeutic benefit of higher rentosertib dosages. FVC evaluations demonstrated a mean change from baseline of -20.3 ml in the placebo group, -27.0 ml in the 30 mg QD group, +19.7 ml in the 30 mg BID group, and +98.4 ml in the 60 mg QD group. Notably, patients not concurrently on SOC medications and taking 60 mg rentosertib QD showed FVC increases of +187.8 ml. Conversely, patients concurrently taking 60 mg rentosertib QD with either nintedanib or pirfenidone did not exhibit significant changes in FVC.
Regarding other lung function metrics, changes in DLCO and FEV1 were relatively small and similar across treatment groups. The impact on general quality of life (QOL) metrics was largely inconclusive, although modeling indicated a significant improvement in the Leicester Cough Questionnaire (LCQ) scores for patients receiving 60 mg rentosertib QD.
Importantly, three patients (16.7%) in the 60 mg rentosertib QD group experienced an acute exacerbation of IPF (AE-IPF), requiring hospitalization, compared to one patient (5.9%) in the placebo group (not hospitalized). The paper notes that a 12-week study is a short timeframe to capture such long-term events.
Conclusions
The present phase 2a clinical trial demonstrates that rentosertib was generally safe and well-tolerated over 12 weeks, with overall TEAE rates similar to placebo, although treatment-related AEs were more common with rentosertib. Serious treatment-related AEs were rare. The trial also showed promising efficacy signals, particularly an FVC increase of +98.4 ml in the 60 mg QD group (and up to +187.8 ml FVC increase in those not on SOC antifibrotics in this dosage group). These findings suggest the drug's potential as a novel therapeutic agent for IPF and warrant further research in larger, longer-term studies.
The study authors acknowledge limitations, including the small cohort size per arm, the geographical and demographic homogeneity of participants (all residents of China of similar race), and the short follow-up period, which restricts the assessment of long-term safety and efficacy.
Notably, this AI-discovered TNIK inhibitor highlights the potential of generative AI-driven drug discovery in pharmacological research and development, suggesting that AI can significantly enhance the efficiency of discovering new medicines.
Written by
Hugo Francisco de Souza is a scientific writer based in Bangalore, Karnataka, India. His academic passions lie in biogeography, evolutionary biology, and herpetology. He is currently pursuing his Ph.D. from the Centre for Ecological Sciences, Indian Institute of Science, where he studies the origins, dispersal, and speciation of wetland-associated snakes. Hugo has received, amongst others, the DST-INSPIRE fellowship for his doctoral research and the Gold Medal from Pondicherry University for academic excellence during his Masters. His research has been published in high-impact peer-reviewed journals, including PLOS Neglected Tropical Diseases and Systematic Biology. When not working or writing, Hugo can be found consuming copious amounts of anime and manga, composing and making music with his bass guitar, shredding trails on his MTB, playing video games (he prefers the term ‘gaming’), or tinkering with all things tech.