Why do men see more dramatic results from the keto diet? Explore the latest science on how hormones and metabolism shape weight loss success for men and women.
 Study: Sex differences in ketogenic diet: are men more likely than women to lose weight? Image Credit: Tatiana Bralnina / Shutterstock
Study: Sex differences in ketogenic diet: are men more likely than women to lose weight? Image Credit: Tatiana Bralnina / Shutterstock
In a recent study published in the journal Frontiers in Nutrition, researchers reviewed sex differences in the efficacy of the ketogenic diet (KD) for weight loss, revealing that men show significantly greater weight loss than women under identical KD protocols. For example, in a 45-day clinical trial, men lost an average of 11.63% of body weight compared to 8.95% in women.
Obesity is a significant public health concern worldwide and is also closely related to cardiovascular disorders, diabetes, and cancers. In 2020, 2.6 billion people aged over five were overweight or obese, and this figure is estimated to exceed four billion by 2035. Dietary interventions for obesity have become a focal point in recent years, with various weight loss strategies being promoted.
The traditional low-fat diet is widely used, but it may promote excessive carbohydrate intake, which could exacerbate weight concerns and lipid abnormalities. In contrast, KD is an ultra-low-carbohydrate, moderate-protein, and high-fat dietary approach. KD induces a state of ketosis, in which the body uses ketone bodies as its primary energy source instead of glucose.
As such, KD has gained popularity as an effective weight loss strategy. Despite the significant weight loss associated with KD, notable sex differences persist. The present study reviewed the literature on the mechanisms of KD in obesity treatment and the sex differences resulting from interactions between hormones and innate factors.
Mechanisms underlying KD
Only minimal ketones are produced under standard dietary conditions. However, KD tricks the body into mimicking a fasting state, and carbohydrate scarcity leads to the accumulation of acetyl-CoA. This triggers the liver into an overdrive, producing excess ketones, such as acetoacetate, acetone, and β-hydroxybutyrate, byproducts of fat metabolism.
Further, the blood-brain barrier (BBB) restricts the brain’s energy sources to ketones and glucose. During fasting, ketones account for 25% to 75% of the brain's energy demands. Thus, KD can maintain regular brain energy supply and peripheral blood glucose levels, promote fat breakdown, and reduce lipogenesis. KD promotes weight loss through various mechanisms.
KD suppresses appetite by elevating peptide neurotransmitters (e.g., glucagon-like peptide-1) and decreasing appetite-regulating hormones (e.g., ghrelin and cholecystokinin), reducing food intake. Furthermore, KD promotes the breakdown of visceral fat, depleting liver glycogen storage and reducing visceral fat accumulation. KD also alters the gut microbiota function, which exhibits sex-specific variations such as a higher abundance of fat-metabolizing bacteria like Bacteroidetes in males, reducing short-chain fatty acid production, which impacts the gut-brain axis signaling.
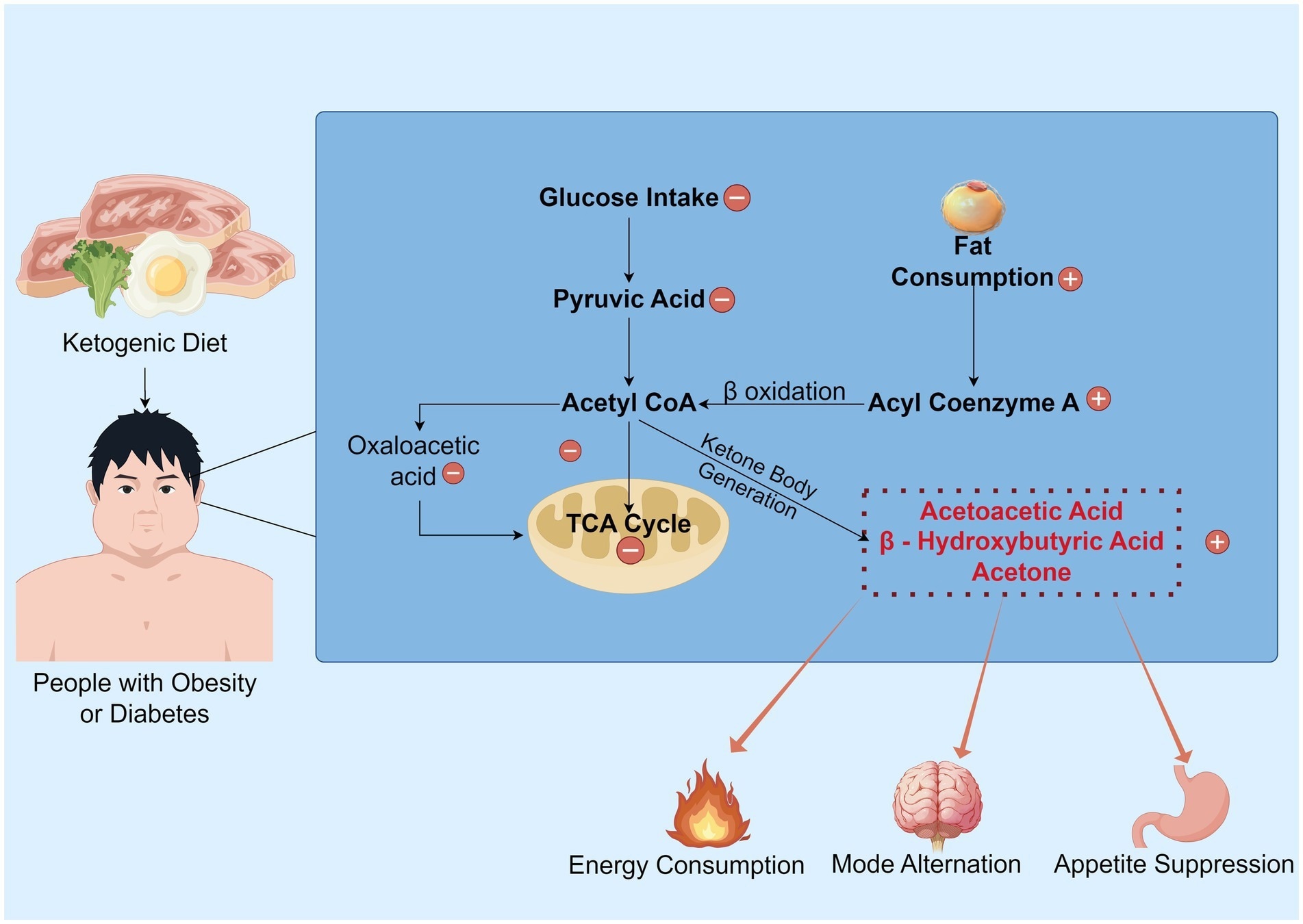 The overview of the KD’s mechanism. Under normal metabolic conditions, glucose serves as the primary energy substrate and is metabolized into pyruvate. This pyruvate is then converted to acetyl CoA, generating oxaloacetate, which enters the TCA cycle to produce ATP. However, under ketogenic dietary states, the synthesis of oxaloacetate is restricted, impeding the normal progression of the TCA cycle. Consequently, a substantial consumption of fat occurs, with processes such as fatty acid activation and β-oxidation generating acetyl-CoA, which promotes the production of ketone bodies. Subsequently, this ketogenesis process yields energy and inhibits appetite, while also shifting the brain to a “fat-fueled” energy mode.
The overview of the KD’s mechanism. Under normal metabolic conditions, glucose serves as the primary energy substrate and is metabolized into pyruvate. This pyruvate is then converted to acetyl CoA, generating oxaloacetate, which enters the TCA cycle to produce ATP. However, under ketogenic dietary states, the synthesis of oxaloacetate is restricted, impeding the normal progression of the TCA cycle. Consequently, a substantial consumption of fat occurs, with processes such as fatty acid activation and β-oxidation generating acetyl-CoA, which promotes the production of ketone bodies. Subsequently, this ketogenesis process yields energy and inhibits appetite, while also shifting the brain to a “fat-fueled” energy mode.
Sex disparities in KD-related weight loss
While studies have not systematically examined the genetic underpinnings of KD-induced sex disparities, literature suggests that these differences may be intricately linked to neurotransmitter levels, genetic factors, intermediate phenotypes, and individual sensitivity to environmental stimuli. Specifically, KD reduces the levels of neurotransmitters, including serotonin, dopamine, and norepinephrine, which affect feeding behaviors.
Catecholamines also inhibit appetite and decrease food intake, helping control calorie intake. Males accumulate fat centrally (visceral fat), which is more readily metabolized under KD conditions, while females store fat subcutaneously. This sex-specific disparity in fat distribution could be linked to the effects of norepinephrine on regional adipose tissue, which is influenced by differing densities and affinities of adrenergic receptors, accounting for variations in KD’s weight loss effects between females and males. Further, the differential response to KD between sexes may be attributed to estrogen, which can increase the sensitivity of α-adrenergic receptors that inhibit fat breakdown.
A study showed that male murine models on KD achieved weight loss and glycemic control, whereas their female counterparts had a slight weight gain, delayed onset of insulin resistance, and compromised glucose tolerance. However, eliminating endogenous estrogen production improved glycemic control and decreased adiposity, comparable to males. Furthermore, testosterone plays a crucial role in the metabolism of proteins, fats, and carbohydrates.
Evidence suggests that testosterone enhances norepinephrine-induced lipolysis in isolated adipocytes from male rats by increasing the number of β-adrenergic receptors that promote fat breakdown. Visceral fat converts testosterone into estrogen in males. As such, an increase in body fat percentage tends to increase estrogen levels in males, while testosterone levels decrease. A recent clinical study reported that overweight males benefit from KD through increased testicular hormone profiles, elevated testosterone/SHBG levels, and reduced obesity markers.
For premenopausal women, the menstrual cycle introduces another layer of complexity. The review highlights that during the luteal phase, elevated progesterone levels can impair insulin sensitivity and increase carbohydrate cravings, making it more difficult to achieve and maintain the state of ketosis necessary for the diet to be effective.
Furthermore, sex disparities exist in immediate energy sources in resting and postprandial states. Females tend to incorporate postprandial free fatty acids (FFAs) into triglycerides, storing fat and using carbohydrates as an energy source. In contrast, males generate energy through FFA oxidation, storing carbohydrates as glycogen. As such, females following KD tend to store fat and face difficulties in fat consumption and mobilization.
KD is also reported to be effective for muscle growth. A randomized controlled trial found that individuals following KD for six weeks gained more muscle than those on a regular diet. Notably, another study found that KD may adversely affect muscle fatigue in young, healthy females, potentially influencing their perception of fatigue. This suggests that the adverse effects of KD on muscle endurance in females may influence the efficacy of weight loss. Finally, differences in brain regulation, such as the reduced potency of the energy-regulating neuropeptide POMC in female mice, also suggest a neurobiological basis for the differing outcomes.
Concluding remarks
Taken together, obesity manifests differently in females and males, and sex differences influence the effectiveness of KD in obesity treatment. Current evidence suggests that KD is most effective in males, followed by postmenopausal females, with its efficacy limited in premenopausal females. These differences could be attributed to genetics, sex hormones, the menstrual cycle, neurotransmitters, neural regulation in energy-expending brain regions, gut microbiota, and immunity. Overall, the study offered insights for improving weight loss strategies and facilitating personalized preventive and therapeutic measures. The authors note that more research in ethnically diverse populations is needed to validate these findings.