Aug 28 2018
Bioinspired enzyme model with a redox switch
Coupled oxygen transfer and electron transfer reactions that use cofactors are enzymatic reactions of crucial significance to all lifeforms from bacteria to vertebrates. In the European Journal of Inorganic Chemistry, scientists have introduced a model for the enzyme sulfite oxidase. It is based on a molybdenum complex whose special ligands can be oxidized to allow coupling of oxygen transfer to a substrate with an intramolecular electron transfer. Intermediates containing molybdenum(V)-ion play a critical role.
Many enzymes do not function alone; they require cofactors—non-protein components that play a role in the transfer of electrons. These often involve metal ions, such as the iron in cytochromes or the trace element molybdenum, which is found in molybdenum oxotransferases, enzymes that transfer oxygen atoms to substrates. An important representative of the latter is sulfite oxidase, which oxidizes toxic sulfite to make sulfate. The oxygen transfer involves a change in the oxidation state of the molybdenum from tetravalent (MoIV) to pentavalent (MoV). The electrons involved are removed by way of cytochromes and used for the production of ATP. Pentavalent molybdenum (MoV) seems to be an intermediate in the reaction.
Researchers working with Katja Heinze at the University of Mainz have now developed a conceptual approach for stabilizing and spectroscopically characterizing the predicted intermediates in the reactions of Mo-containing oxotransferases by using model systems. They use a method that is also used in controlling catalysts: they attach a molecular “switch” with a controllable oxidation state.
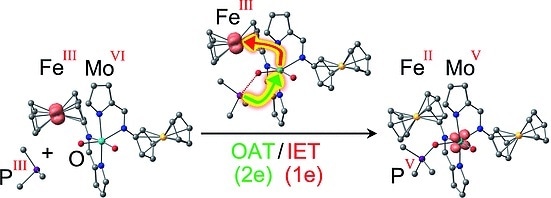
The researchers elected to use the ferrocene/ferrocenium redox pair, in which an iron atom is sandwiched between two negatively charged, aromatic, five-membered carbon rings. The iron ion can switch between a state with a twofold positive charge (FeII, ferrocene) and a threefold positively charged, oxidized state (FeIII, ferrocenium). This is analogous to the naturally occurring cytochrome cofactors, which may also contain FeII or FeIII. The researchers attached two of these ferrocene/ferrocenium “switches” to a molybdenum complex as a model for the active center of a sulfite oxidase. The molybdenum is in the VI oxidation state and has two oxygen atoms attached by double bonds. Reaction with an organic phosphane (a phosphorus-hydrocarbon molecule) as a model oxygen-acceptor substrate occurs at variable rates, depending on the charges on the “switches”, because the redox status of the ferrocenyl substituents modulates the energy barriers for certain intermediates. This results in a variety of adducts between the Mo complex and the phosphane.
The most interesting versions are those with two FeIII ions or one FeII and one FeIII ion: In these cases, the mechanism is no longer a simple transfer of one oxygen atom to the substrate, as for the FeII/ FeII version. Instead it is coupled to an intramolecular electron transfer, possibly conceptually similar to that for natural sulfite oxidases. During the oxygen transfer reaction, an electron is transferred from the molybdenum to one of the FeIII ions, resulting in a charge distribution of either FeII FeIII MoV or FeII FeII MoV. For the latter version, the researchers were able to use electron spin resonance spectroscopy to unambiguously detect the MoV intermediate FeII FeII MoV with the oxidized substrate (the product) still bound to it. The coupling of the oxygen transfer to an intermolecular electron transfer lowers the activation barrier for this reaction step and simultaneously stabilizes the MoV intermediate, as predicted by the computer models.
The researchers propose to use this new system as a model for the oxygen atom transfer and intramolecular electron transfer steps in sulfite oxidases, and hope to use this new concept to develop analogs for further enzymatic reactions with coupled electron transfer. In the future, it also seems possible to extend this concept to switchable catalytic reactions that have no natural prototype.