The skyrocketing number of cases due to the coronavirus pandemic has taken a massive toll on the health systems in many countries. Since the advent of the coronavirus disease (COVID-19), hundred of research teams are racing to find an effective vaccine and treatment to combat the deadly infection. Now, a team of researchers in Hong Kong suggests that combining three existing antivirals may help treat COVID-19 patients.
The team has found that patients experiencing mild symptoms caused by the novel coronavirus recover faster if they are treated with a triple-drug cocktail soon after symptoms appear.
The results of the randomized trial for COVID-19 treatment with a triple-drug combination, including interferon beta-1b, lopinavir-ritonavir, and ribavirin, has shown that the combination is safe and effective in lowering severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral shedding duration. Viral shedding is when the virus is detectable and potentially transmissible.
The drug trial
The trial, which involved 127 patients, compared the effect of the triple-drug combination, including the anti-HIV therapy lopinavir-ritonavir, the multiple sclerosis treatment interferon-beta, and the hepatitis drug ribavirin, with a control group that was given just lopinavir-ritonavir.
The patients were admitted to one of six public hospitals with laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection from Feb. 10 to Mar. 20.
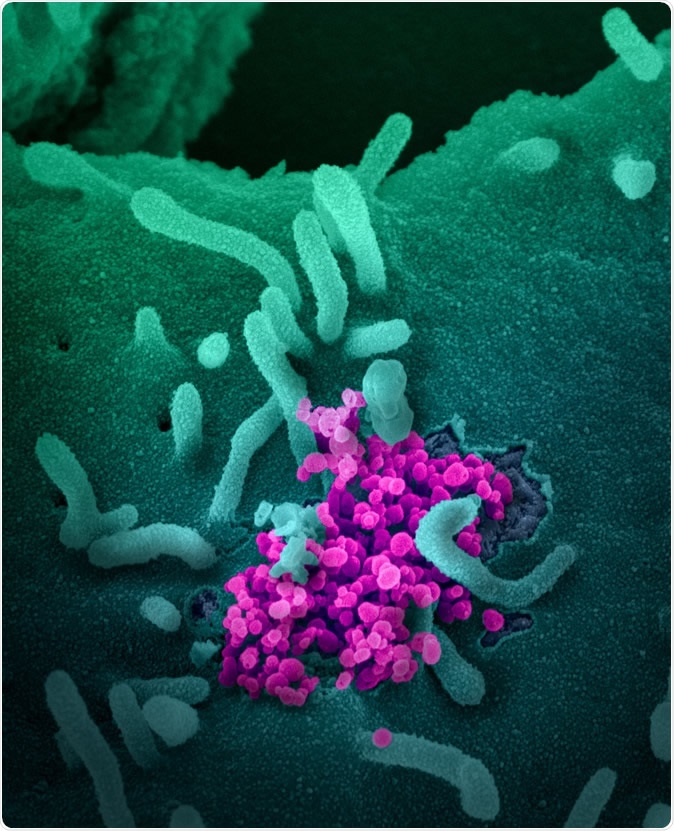
Novel Coronavirus SARS-CoV-2 This scanning electron microscope image shows SARS-CoV-2 (round magenta objects) emerging from the surface of cells cultured in the lab. SARS-CoV-2, also known as 2019-nCoV, is the virus that causes COVID-19. The virus shown was isolated from a patient in the U.S. Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Credit: NIAID
In the past, a combination of antivirals was used in patients, such as those with flu. Previous experience with a combination of antivirals showed promise in treating patients with influenza, showing that it is more effective than using a single drug. Guided from previous studies, the researchers hypothesized that using a triple combination of antivirals could be a potential therapeutic approach for COVID-19.
The patients in the study all had mild to moderate symptoms and were given the combination drug within seven days of testing positive for SARS-CoV-2 infection. The researchers believe that treating the patients early in the disease process is more effective than those who already developed severe symptoms.
Promising results
The patients who received the drug combination tested negative for SARS-CoV-2 after seven days, while those who just took the HIV drugs, had an average of 12 days before testing negative for the virus.
Secondary outcomes supported the findings, showing that the clinical improvement was better in the triple antiviral group. The patients experienced the alleviation of symptoms on an average of four days, compared to eight days in the two-drug combination group. Further, those who received the triple combination drugs had a shorter hospital stay of nine days, compared to 14.5 days in the control group.
“Our trial demonstrates that early treatment of mild to moderate COVID-19 with a triple combination of antiviral drugs may rapidly suppress the amount of virus in a patient’s body, relieve symptoms, and reduce the risk to health-care workers by reducing the duration and quantity of viral shedding (when the virus is detectable and potentially transmissible). Furthermore, the treatment combination appeared safe and well-tolerated by patients,” Professor Kwok-Yung Yuen, from the University of Hong Kong and study lead researcher, said.
During the treatment, a patient in the control group developed a serious side effect affecting the liver, which prompted the discontinuation of the treatment. In the treatment group, no adverse event was reported, and no patient died during the study duration. There are fewer side effects, such as nausea, fever, and diarrhea.
The researchers said that despite promising results of the trial, which is published in The Lancet, the combination should be tested and confirmed in a more extensive phase 3 trial.
“This study presents a step towards finding a much-needed therapy for SARS-CoV-2. However, as the authors acknowledge, future studies to examine the efficacy of interferon beta-1b alone or in combination with other drugs to treat severe or critically ill patients with confirmed COVID-19 compared with placebo are warranted,” Dr. Sarah Shalhoub, from Western University in Canada who was not involved in the study, said.
The race for treatments and vaccines for the coronavirus pandemic is game on since the number of infections topped 4.17 million, and the death toll over 286,000. The United States is the hardest-hit nation, with more than 1.34 million infections and more than 80,000 deaths.
Source:
Journal reference:
- Hung, I., Lung, K.C., Tso, E.Y., Liu, R., Chung, T.W., and Chu, M.Y. et al. (2020). Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. The Lancet. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31042-4/fulltext