A recent study by the US researchers highlights the value of the human organ chip technology as a more robust and physiologically relevant platform for rapid drug repurposing, and suggest that amodiaquine may be used to prevent the infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The paper is currently available on the bioRxiv* preprint server.
The ongoing coronavirus disease (COVID-19) pandemic caused by SARS-CoV-2, as well as potential future ones caused by other coronaviruses and influenza viruses, represent impending dangers and major public health concerns.
The most rapid way to tackle such pandemic challenges would be to repurpose existing drugs approved for other medical indications as antivirals. And while scientists worldwide are striving to do this for the COVID-19 pandemic since it started, thus far, these ventures have been haphazard, relying mostly on the results generated using cell lines in laboratory conditions.
US National Institutes of Health (NIH), together with some other agencies, was farsighted in recognizing that human organ-on-a-chip (Organ Chip) microfluidic culture technology could be useful in confronting potential biothreat challenges, recapitulating organ physiology, disease states, and treatment responses with high fidelity.
This is why a US research group, led by Dr. Longlong Si from Harvard University, aimed to explore whether this technology can be utilized to ascertain the efficacy of potential antiviral therapeutics in our fight against COVID-19.
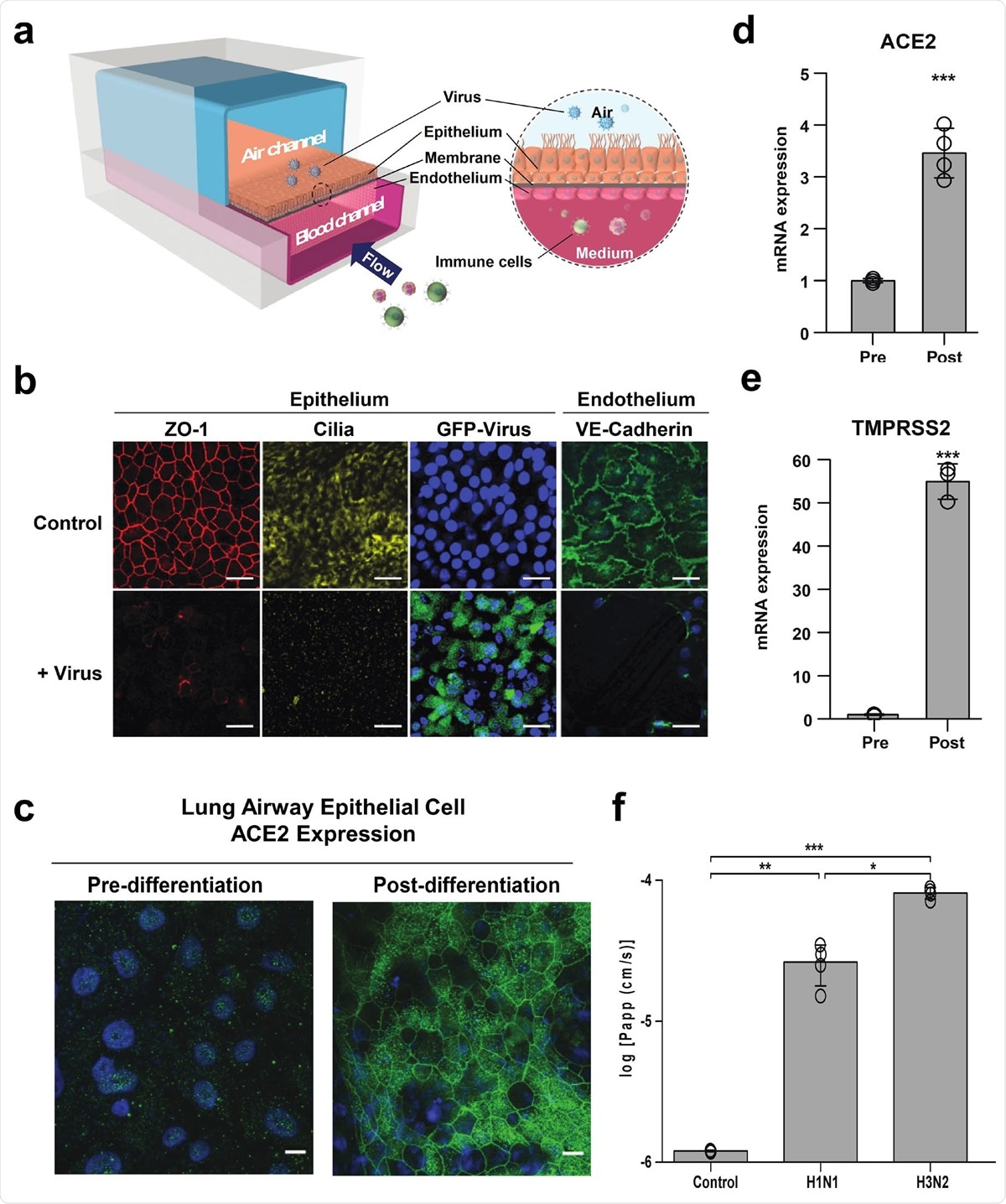
Characterization of the human Airway Chip and its infection with influenza virus. (a) Schematic diagram of a cross-section through the Airway Chip. (b) Immunofluorescence micrographs showing the distribution of ZO1-containing tight junctions and cilia in the epithelium and VE-cadherin-containing adherens junctions in the endothelium of the Airway Chip in the absence (Control) or presence (+ Virus) of infection with GFP-labeled influenza PR8 (H1N1) virus (MOI = 0.1) for 48 h (blue, DAPI-stained nuclei; bar, 50 μm). (c-e) Immunofluorescence micrographs showing the expression of ACE2 receptor (c) and fold changes in mRNA levels of ACE2 (d) and TMPRSS2 (e) in the well-differentiated primary human lung airway epithelim on-chip (Post) versus the same cells prior to differentiation (Pre). (f) Increase in barrier permeability as measured by apparent permeability (log Papp) within the human Airway 931 chip 48 h post-infection with PR8 (H1N1) or HK/68 (H3N2) virus (MOI = 0.1) compared to no infection (Control).

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Devising a human airway chip culture
By exploiting their rich expertise in microfluidic tissue chips, these scientists initially applied developed mature physiologic microfluidic small airway bilayer model in order to assess treatments for influenza infections, but quickly pointed their eye to COVID-19.
More specifically, this revolutionary model is based on differentiated human lung airway epithelial basal stem cells. It is comprised of a pulmonary endothelial (microvascular) cell bilayer with a flow through the vascular channel, as well as a well-developed air-liquid interface within the epithelial channel.
Other techniques used in this research endeavor were immunofluorescence microscopy, quantitative reverse transcription-polymerase chain reaction (RT-qPCR), plaque formation assay, pseudotyped virus production, plasmid expression, and finally, hamster efficacy studies.
Rapid identification of repurposable drugs
"Taken together, these data show that human Organ Chips, such as the Lung Airway Chip, can be used to rapidly identify existing approved drugs that may be repurposed for pandemic virus applications in crisis situations that require accelerated development of potential therapeutic and prophylactic interventions," summarize study authors.
It was shown that the anticoagulant drug nafamostat (a wide spectrum protease inhibitor) substantially extends the current treatment time window of oseltamivir from two to four days after infection by influenza virus, with potential clinical relevance – especially considering that most patients do not begin treatment until days following the infection.
Similarly, the researchers successfully singled out amodiaquine as a putative treatment option for COVID-19 that works both in the laboratory condition and in the human body. Amodiaquine is a highly efficacious anti-malarial drug-related to chloroquine and hydroxychloroquine.
From bench to bedside
Given the alarming rate at which the COVID-19 pandemic is spreading, clinicians have to seriously appraise all possible risks and benefits of using any existing approved drug as a novel treatment option. Furthermore, specific patient populations have to be taken into account before commencing any clinical trial in their respective local communities.
"Our findings raise the possibility that amodiaquine could be explored as a chemoprophylaxis therapy to prevent the spread of COVID-19 and help people return to their workplace by treating healthy patients for 3 days, which could then offer protection for an additional 2 weeks", say study authors in this bioRxiv paper.
Nonetheless, further tests are needed to gauge the effectiveness of amodiaquine for treating cells and tissue infected with SARS-CoV-2 in vitro and in vivo, rather than merely preventing the infection.
And when discussing the predictive behavior of each model, a direct comparison of drug efficacy against the putative virus in the Lung Airway Chip versus animal models would be helpful and more insightful. In the meantime, this type of approach may lower the barrier for a potential drug to reach human clinical trials.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.