Since the coronavirus disease 2019 (COVID-19) was first detected in December 2019 in Wuhan, China, many different variants of its causative virus – severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – have emerged. Through increased viral fitness, some of these have risen to predominance over earlier variants in a remarkably short period of time. New research, by a team of scientists at major institutions in France, examines the reasons behind this phenomenon in one such variant of concern (VOC), the so-called UK variant.
Background
Also known as the B.1.1.7 variant, the UK variant reproduces up to 90% more rapidly to produce secondary cases and may soon become the dominant lineage worldwide. The underlying biology responsible for this is unclear but may include a higher viral load and a longer infectious period.
Viral shedding is closely related to infectious potential, and is known to be higher in B.1.1.7, which also shows a longer infectious period and a higher viral load. The current study focuses on the viral infectivity of this strain compared to others.
Study details
The researchers examined the virus and cytokine loads in over 400 nasopharyngeal (NP) swabs that tested positive for SARS-CoV-2 by the reverse transcriptase-polymerase chain reaction (RT PCR). This test looks for genetic material – namely, viral ribonucleic acid (RNA).
The swabs were from individuals infected by both UK and non-UK strains and were obtained at different days from symptom onset.
The presence of the viral antigen was evaluated by a lateral flow antigen rapid diagnostic test (RDT). In addition, antibodies to the virus, both IgG and IgA, were measured in the samples. Finally, the titers of 48 cytokines were assessed.
Viral infectivity was measured by allowing the virus to infect cells expressing the angiotensin-converting enzyme 2 (ACE2) that binds the viral spike antigen, allowing viral entry. The cells were tagged with a fluorescent marker that lit up when the cells fused together to form syncytia, as expected following infection.
The cells were engineered to express TMPRSS2, an enzyme that increases susceptibility to this virus by priming the spike glycoprotein.
Two sample sets of NP swabs were tested, one with 200 samples with PCR cycle thresholds (Ct) <40, collected in the period before the UK variant emerged. These sequences were assumed to belong to non-UK lineages.
The second came from a later period, with ~23- samples that had Ct < 33, and seven with Ct > 33. This consisted of both UK and non-UK strains, at 70 and ~160 each.
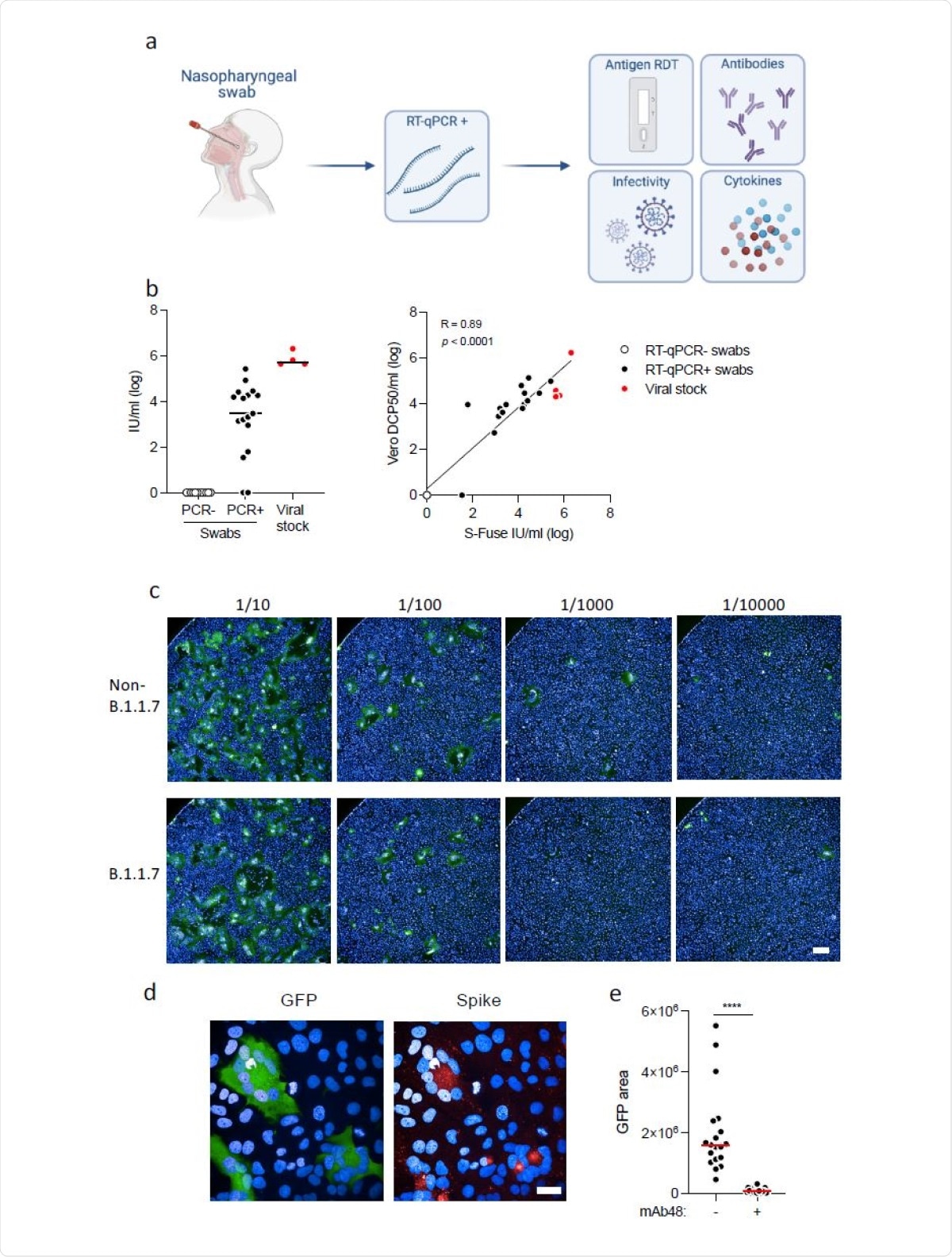
Detection of infectious virus, antibodies and cytokines in nasopharyngeal swabs. a. Study design. A retrospective series of 427 RT–qPCR+ nasopharyngeal swabs from COVID-19 patients, harboring non-B.1.1.7 or B.1.17 variants, was analyzed. Four tests were performed: a lateral flow antigen rapid diagnostic test (RDT), anti-SARS-CoV-2 IgG and IgA were measured with the flowcytometry based S-Flow assay, infectious virus was titrated with the S-Fuse assay, and 46 cytokines were quantified in a subset of 202 swabs (70 non-B.1.1.7 and 132 B.1.1.7 samples) by Multiplex or Simoa assays. b. Titration of infectious SARS-CoV-2 in the swabs. Left panel: Titration was performed with S-Fuse-T cells, which become GFP+ after infection. S-Fuse-T cells were exposed to serially diluted swabs (10-1 to 10-5) or to purified viral stocks (red dots) as a control. 12 RT–qPCR- (white dots) and 17 RT–qPCR+ (black dots) samples were first analyzed. An infectious titer was calculated in Infectious Units (IU)/ml, after automatic scoring of the area of GFP+ cells at each dilution. Right panel: correlation between titers measured in Vero cells (in DCP50/ml) and S-Fuse-T cells (in IU/ml). c. Representative images of S-Fuse-T cells exposed to the indicated dilutions of nasopharyngeal swabs. Samples from one non-B.1.1.7-infected and one B.1.1.7-infected individuals are shown. Scale bar: 400µm. d. GFP (green) and S (red) expression in S-Fuse-T cells exposed to one infectious swab analyzed by immunofluorescence. The Hoechst dye (blue) stains the nuclei. Scale bar: 40 µm. e. Neutralization of infectious virus by mAb48. Swabs (n=19) were preincubated 30 min at RT with mAb48 (1 µg/ml) and added to S-Fuse-T cells. Result from one representative experiment out of 3 is shown. A Wilcoxon paired t-test was performed **** p<0.0001.
What were the results?
Both variants had Ct values below 22. In those carrying infectious viruses, the Ct was 17 and 19 for the UK and non-UK strains, respectively. Viral shedding occurred at a median of 2 days after symptoms began.
In both cases, antibodies were detected only after 5 days, but before 10 days, in most cases, in NP swabs. Thus, the presence of antibodies seems to prevent the further presence of infectious virus, limiting viral shedding. This corroborates earlier findings that impaired antibody production allows prolonged viral shedding.
The results in the first pre-B.1.1.7) samples set showed that samples varied widely in their infectivity. Over half had detectable infectious virus, though the viral loads ranged from 0 to 105- 6Infectious Units/mL (IU)/ml). The median titer was 1000 IU/mL.
The higher the infectious titer, the lower was the SARS-CoV-2 RNA load, as assessed by PCR Ct values. Conversely, the antigenic RDT was positive in over 90% of samples containing viable virus. That is, a positive RDT detected 93% of samples with detectable viable virus. A negative RDT would have 78% specificity, implying it would not report 22% of the negative samples correctly.
RDT correlates with infectious virus content
Ct values were lower, at a median of 22, in samples with a positive RDT, compared to 31 in the positive cohort. This indicates that samples containing infectious virus have a more than 600-fold higher viral RNA content.
Two out of three individuals were most likely to be infectious within the first few days of symptom onset, at a median of 2 days, when the Ct values were below 22.
In the second sample set, RDT was positive in about 72-73% of samples, for both variants. Similarly, infectious virus was detectable in about 40-42% of samples with both variants. The presence of infectious viral particles predicted a positive RDT in 97% of cases.
Extent of viral shedding
Viral shedding is known to begin up to six days before symptoms begin, but viral loads fall steadily thereafter. On day 2, detectable virus was present in similar fractions of the UK and non-UK variant groups.
However, the UK variants tended to fall faster, perhaps indicating that peak infectivity occurs before symptoms began. This could not be confirmed as no pre-symptomatic samples were available and may be the result of somewhat lower viral titers, overall, with this variant.
Cytokine responses
In both sample types (containing the UK and non-UK variants), cytokines were evaluated, showing that some were increased in critical COVID-19 patients compared to those without such severe illness. For some cytokines, such as IFNa2, the levels were lower as Ct values rose for both UK and non-UK variants.
Further analysis revealed that this increase in the number of cytokines occurred mainly in patients infected with the UK strain. These included beta-interferon, granzyme B, several interleukins such as IL-10, IL-13 and IL-15, and the growth factor GM-CSF that stimulates the growth of white cells other than lymphocytes in the blood.
What are the implications?
The study used a novel cell culture with a reporter system with enhanced susceptibility to SARS-CoV-2 infection via TMPRSS2 expression by forming fluorescent syncytia within 24 hours. This assay is also more convenient since it is partly automated, and can be applied to different clinical samples.
In addition to infectious virus, RT PCR, RDT, antibody titers and cytokine concentrations were also measured to visualize how long infectivity persists in NP swabs, with either UK or non-UK variants. The determinants of such shedding were also evaluated.
The findings show that about half the patients were infectious at the time of sample collection. This is in contrast to an earlier study that shows less than 10% infectivity in hospitalized patients, though another, presumably less efficient, assay was used for viral detection.
However, viable virus is shed only until antibody production begins, and long-term shedding may comprise viral remnants rather than infectious viruses. Viable virus particles were mostly detected within ten days, with a few samples going up to 14 days, indicating a longer period of infectivity than earlier observed.
This contrasts with earlier studies, where live virus has not been observed beyond 8-9 days, though this may be due to the low sensitivity of the cell-based assay method used. Exceptions to this were in patients with impaired immunity and one patient with severe COVID-19.
Though the different variants failed to show any marked differences in peak infectivity and viral shedding, the B.1.1.7 variant appears to show a faster decrease in infectivity over time.
Most importantly, higher levels of cytokines were obvious in samples containing B.1.1.7. This indicates that this variant may produce a more intense inflammatory reaction leading to more severe disease.
This reflects the findings of some animal studies, where IL-6, IL-10 and IFN-γ were much higher in hamsters infected with the UK variant but not with three other strains.
Further studies should look into the correlation between cytokine levels and transmissibility or viral shedding. Also, the comparability of NP cytokines with levels in blood samples should be examined, as immunologic studies suggest that immune responses to the infection tend to be localized to the infected tissue.
Larger studies, including asymptomatic patients, will help to assess whether these findings remain valid, since these individuals are responsible for most of the viral spread at the community level.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Monel, B. et al. (2021). Release of infectious virus and cytokines in nasopharyngeal swabs from individuals infected with non-B.1.1.7 or B.1.1.7 SARS-CoV-2 variants. medRxiv preprint. https://doi.org/10.1101/2021.05.20.21257393, https://www.medrxiv.org/content/10.1101/2021.05.20.21257393v1
- Peer reviewed and published scientific report.
Monel, Blandine, Delphine Planas, Ludivine Grzelak, Nikaïa Smith, Nicolas Robillard, Isabelle Staropoli, Pedro Goncalves, et al. 2021. “Release of Infectious Virus and Cytokines in Nasopharyngeal Swabs from Individuals Infected with Non-Alpha or Alpha SARS-CoV-2 Variants: An Observational Retrospective Study.” EBioMedicine 73 (November). https://doi.org/10.1016/j.ebiom.2021.103637. https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(21)00430-8/fulltext