Researchers in the United States, the UK and France have shown that using antiviral monoclonal antibodies in combination with vaccination can be expected to suppress the transmission of severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) and reduce the burden of coronavirus disease 2019 (COVID-19) more than vaccination alone.
The team’s modeling simulations suggested that the near-term use of monoclonal antibodies (mAbs) for early treatment and post-exposure prophylaxis (PEP) in combination with vaccination would substantially reduce SARS CoV-2 transmission and COVID-19 mortality.
Mohamed Kamal from Regeneron Pharmaceuticals in Tarrytown, New York and colleagues say that allocating mAbs solely as PEP or targeting those aged 65 years and older provided the most significant incremental benefits relative to vaccination alone.
The use of rapid diagnostic testing and increasing the supply of mAbs further reduced infections and mortality – by as much as two-fold – compared with vaccination alone.
Furthermore, the use of mAbs continued to provide additional benefits, even as the proportion of the population being vaccinated increased. The researchers say this emphasizes the potential utility of mAbs, even as herd immunity is approached.
“These results may help guide resource allocation and patient management decisions for COVID-19 and can also be used to inform public health policy for current and future pandemic preparedness,” writes the team.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Considering a combination of mAbs and vaccination in public health planning
Since the COVID-19 outbreak first began in late December 2019, unprecedented global efforts to develop vaccines against SARS-CoV-2 have led to the authorization and mass roll-out of several vaccines in many countries as a long-term strategy to achieving herd immunity.
Researchers have also been developing mAbs for both the treatment and prevention of COVID-19.
While vaccines induce the development of active immunity over time, mAbs confer immediate passive immunity to protect individuals recently exposed to SARS-CoV-2.
However, the combined use of mAbs and vaccination has not yet been considered in public health strategic planning, say the researchers.
Multiple COVID-19 prevention strategies are ongoing concomitantly, making it challenging to generate real-time, controlled, clinical trial data to understand their combined benefits, they add.
“Mathematical modeling is an important tool to inform decision-making in scenarios where the prospective evaluation of pandemic interventions in concert may not be feasible,” writes the team.
What did the current study involve?
The team developed a model that enabled simulation of various pandemic scenarios relative to a base case that assumed implementation of various non-pharmaceutical interventions (NPIs).
The team used the model to characterize SARS-CoV-2 transmission in the United States during an aggressive phase of the pandemic (October 2020 to April 2021) and simulated the effects of combining mAbs as an active treatment or PEP with a vaccination program.
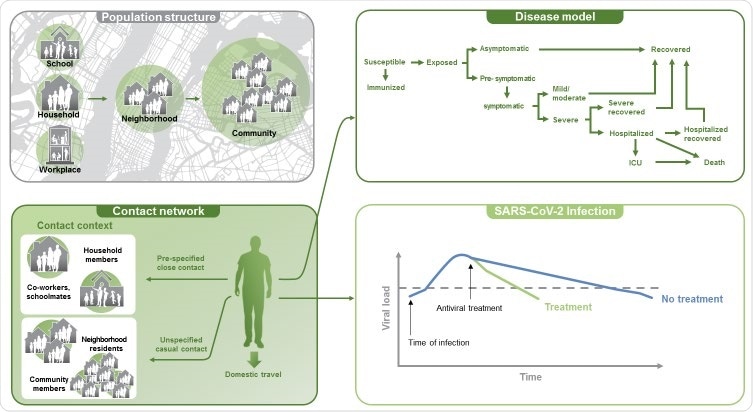
Components of the COVID-19 agent-based model. Development of the agent-based model incorporated 7 modules to allow for simulation of mitigation strategies for the COVID-19 pandemic. Components of the model consisted of the US population structure, a base social network, movement/travel within the US, virus transmission, a disease model, a baseline use of non-pharmaceutical interventions, and pharmaceutical interventions.
In the base case, there were approximately 103 million cumulative infections and 338,000 cumulative deaths over the simulation period.
The roll-out of vaccination to 15% of the US population averted almost 6 million infections and 43,000 deaths, corresponding to a reduction of around 6% for transmission and 13% for mortality.
Specifically targeting people aged 65 and older
Simulations of mAbs used as an active treatment in combination with vaccination showed overall effects that were similar to those of vaccination alone.
When mAbs were used as both an active treatment and PEP, 7% more infections were averted overall, compared with vaccination alone. However, when this strategy was targeted at individuals aged 65 years and older, the number of averted deaths increased by 41%.
The use of mAbs solely as PEP increased the overall number of infections averted by around 17%, relative to vaccination, while simultaneously reducing mortality by 7%.
When this approach was targeted specifically at an older population, mortality was reduced by 29%, although no additional benefit was observed for the number of infections averted.
Combining the mAb and vaccination approaches with rapid testing
Under conditions of rapid testing, using mAbs as both an active treatment and PEP in combination with vaccination averted 20% more infections than vaccination alone, while using mAbs solely as PEP averted 50% more infections. Furthermore, targeting this approach to those aged 65 years or older increased the number of deaths averted by as much as 58% to 66%.
The use of rapid testing combined with a doubling of the mAb supply from 300,000 to 600,000 per month and allocation solely as PEP averted approximately twice the number of infections and reduced mortality by 45%, compared with vaccination alone.
“Pairing such testing with a readily accessible PEP regimen is expected to offer a substantial impact on both disease transmission and clinical outcomes,” says the team.
Furthermore, the simulations showed that the use of mAbs consistently averted substantially more infections and deaths, even as the proportion of the population being vaccinated increased.
“These results emphasize the utility of mAbs, even as herd immunity is approached,” says the team.
What did the team conclude?
The researchers say the findings indicate that the use of antiviral mAbs as a treatment and PEP in combination with vaccination would substantially reduce SARS-CoV-2 transmission and the burden of COVID-19.
“These results may help guide resource allocation and health policy decisions for COVID-19 management and may additionally be applicable to future pandemic preparedness,” they conclude.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.