Researchers reporting on findings from the Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial in the UK are recommending that any therapeutic use of REGEN-COV may be best restricted to individuals without detectable antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The RECVORY Collaborative Group says that among patients hospitalized with coronavirus disease 2019 (COVID-19), REGEN-COV reduced 28-day mortality by about one-fifth in those who were seronegative at baseline.
REGEN-COV comprises two monoclonal antibodies (casirivimab and imdevimab) that bind to different epitopes on the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein. The spike RBD mediates the initial stage of the infection process when it binds to the human host cell receptor angiotensin-converting enzyme 2.
Treatment with REGEN-COV was also associated with a reduced rate of progression to invasive mechanical ventilation or death among SARS-CoV-2 seronegative individuals.
However, “the proportional effect of REGEN-COV on mortality differed significantly between seropositive and seronegative patients,” says Peter Horby from the University of Oxford and colleagues.
No such benefits of REGEN-COV therapy were observed for patients who were positive for anti-SARS-CoV-2 antibodies at baseline.
“Based on our findings, any therapeutic use of REGEN-COV in the hospital setting may be best restricted to seronegative patients,” advises the team.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The progress with monoclonal antibodies so far
The clinical efficacy of monoclonal antibodies (mAbs) in viral infections is thought to be mediated by direct binding to free virus particles and neutralization of their ability to infect host cells.
Following the emergence of SARS-COV-2, mAbs targeting the spike RBD were rapidly isolated from humanized mice and from peripheral B cells of recovered patients.
In the United States, the European Medicines Agency has authorized REGEN-COV for use in patients at high risk of progressing to severe COVID-19 but do not require supplemental oxygen.
In a study of non-hospitalized adults with SARS-COV-2 infection and risk factors for severe COVID-19, REGEN-COV reduced viral load in the upper airway, shortened the time to symptom resolution, and reduced hospitalizations and mortality, compared with placebo.
“However, to date, no virus-directed therapy has been shown to reduce mortality in hospitalized patients with COVID-19, for whom the only treatments so far shown to reduce mortality have been those that modify the inflammatory response,” says Horby and colleagues.
The researchers suspect that the clinical response to antibody-based therapies may be strongest among individuals with early-stage disease or who fail to mount an effective immune response.
“This is supported by evidence of clinical benefit in early disease and evidence that baseline anti-SARS-CoV-2 antibody status may be an important predictor of the effect of anti-spike mAbs on viral load,” writes the team.
What did the current study involve?
The RECOVERY Group evaluated the efficacy and safety of REGEN-COV in patients admitted to hospital with COVID-19.
Between September 18th, 2020, and May 22nd, 2021, 9,785 patients were randomly allocated to receive either usual care plus a single dose of REGEN-COV 8g (casirivimab 4g and imdevimab 4g) or usual care alone.
The study cohort (mean age 61.9 years) included 3,153 (32%) seronegative patients, 5,272 (54%) seropositive patients and 1,360 (14%) patients with unknown baseline antibody status.
The primary outcome was 28-day mortality and secondary outcomes were time to discharge from hospital and the composite outcome of progression to invasive mechanical ventilation or death.
What were the study findings?
Of the study participants, 4,839 received REGEN-COV plus usual care, and 4,946 received usual care alone.
Among the seronegative patients, 396 (24%) of 1,633 patients allocated to REGEN-COV and 451 (30%) of 1,520 patients allocated to usual care died within 28 days.
However, there was no meaningful difference in 28-day mortality among the overall study population.
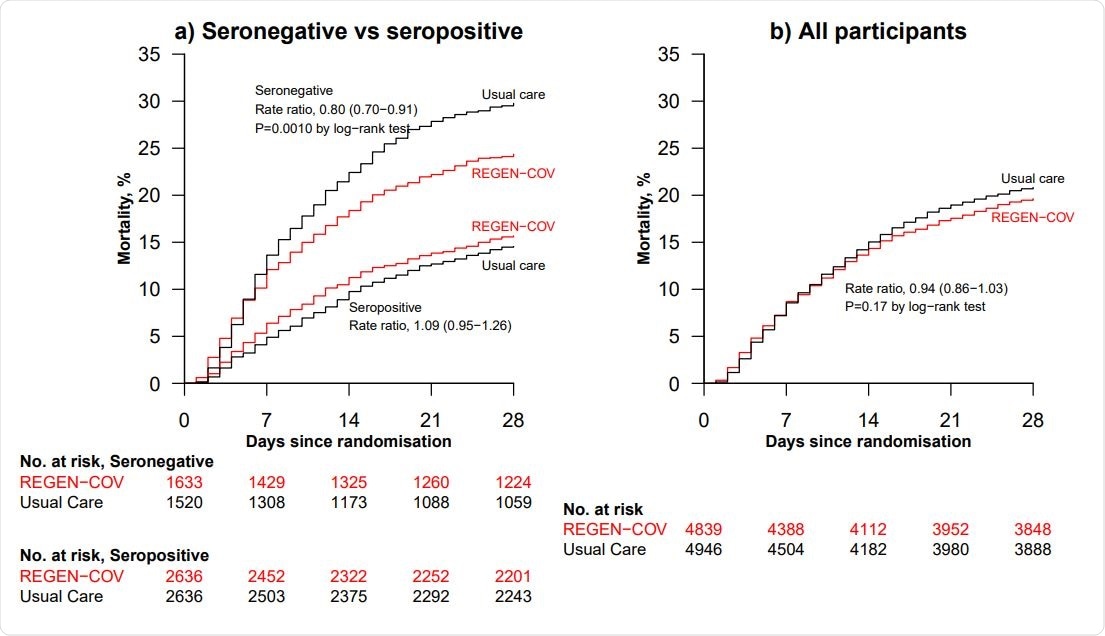
Effect of allocation to REGEN-COV on 28-day mortality (a) in seronegative patients and seropositive patients (b) overall
In an analysis of all patients (irrespective of baseline antibody status), 944 (20%) of 4,839 allocated to REGEN-COV and 1,026 (21%) of 4946 patients allocated to usual care died within 28 days.
Among seronegative patients, allocation to REGEN-COV was associated with a lower risk of progressing to the composite secondary outcome of invasive mechanical ventilation or death than allocation to usual care alone, at 30% versus 37%.
However, again, no significant difference was observed for this outcome in the study population overall (24% versus 25%).
The frequency of fever, sudden hypotension or thrombotic events was marginally higher in the REGEN-CoV versus usual care group, while the frequency of sudden worsening in respiratory status or clinical hemolysis was marginally lower.
What did the authors conclude?
“Based on our findings, any therapeutic use of REGEN-COV in the hospital setting may be best restricted to seronegative patients,” writes the team. “This would require serological testing prior to drug administration.”
“In summary, this large, randomized trial provides the first evidence that an antiviral therapy can reduce mortality in hospitalized COVID-19 patients and the results support the use of REGEN-COV in seronegative patients hospitalized with COVID-19,” concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Horby P, et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomized, controlled, open-label, platform trial. medRxiv, 2021. doi: https://doi.org/10.1101/2021.06.15.21258542, https://www.medrxiv.org/content/10.1101/2021.06.15.21258542v1
- Peer reviewed and published scientific report.
Abani, Obbina, Ali Abbas, Fatima Abbas, Mustafa Abbas, Sadia Abbasi, Hakam Abbass, Alfie Abbott, et al. 2022. “Casirivimab and Imdevimab in Patients Admitted to Hospital with COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial.” The Lancet 399 (10325): 665–76. https://doi.org/10.1016/s0140-6736(22)00163-5. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(22)00163-5/fulltext.