In a study conducted at the University of Washington, USA, the researchers assert that post-exposure prophylaxis with monoclonal antibodies could improve the clinical outcomes and health system costs regarding coronavirus disease (COVID-19). The study is currently available on the medRxiv* preprint server.
The highest impact of the COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been observed in the USA, with more than 39.9 million infections and over 648,000 deaths. Despite sufficient vaccine availability and mass vaccination efforts, the number of new infections is constantly increasing in the country, most probably because of emerging viral variants with higher immunogenicity, vaccine hesitancy, and waning vaccine immunity.
Besides vaccines, monoclonal antibody (mAb) therapies have demonstrated efficacy to prevent infection among unvaccinated individuals with a high-risk exposure to someone with SARS-CoV-2 infection. Unlike vaccines that induce host cell immunity to eliminate the virus, monoclonal antibodies directly bind to viral antigen, leading to faster virus removal from the system. This makes monoclonal antibody therapy attractive prophylaxis to prevent household transmission of SARS-CoV-2. However, a high production cost sometimes limits the large-scale application of antibody-based therapies.
In the current study, scientists have assessed monoclonal antibodies' health benefits and costs as post-exposure prophylaxis.
Study design
The scientists collected information about antibody efficacy from the phase 3, placebo-controlled, household randomized trial of the monoclonal antibody cocktail casirivimab and imdevimab. In the trial, the antibody cocktail was injected subcutaneously to asymptomatic, SARS-CoV-2 negative household members of people with confirmed COVID-19. According to the trial findings, monoclonal antibody therapy reduces the risk of symptomatic COVID-19 by 81%.
From the trial findings, the scientists estimated a secondary infection rate of 7.8%. They used a decision-analytical model to combine the trial results with the population data on household demographic details, the number of COVID-19 cases and demographics, and vaccine coverage to estimate the number of symptomatic COVID-19 cases, hospitalization, mortality, and health system cost of antibody therapy.
Important observations
About 753 participants were treated with the antibody cocktail in the trial, and 752 participants were in the placebo group. The scientists combined the results with COVID-19 case data covering 154,136 individuals, vaccination data covering 1,888 individuals, and household demographic data covering 3,190,040 individuals in 1,394,399 households. Based on the ethnic characteristics of household members, they estimated that about 17%, 20%, 53%, and 11% of unvaccinated household contacts are non-Hispanic Black, Hispanic, non-Hispanic White, and other non-Hispanic ethnic groups, respectively.
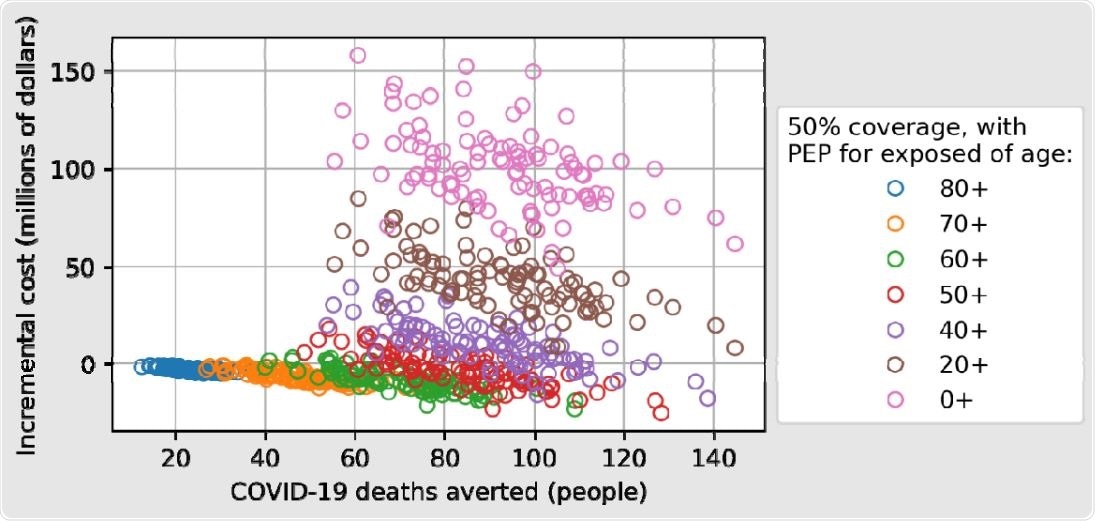
The number of COVID-19 deaths averted increases as the minimum age threshold for receiving PEP is decreased, and the incremental cost (including cost of PEP with mAbs plus cost of COVID-19 hospitalizations) shows the tradeoff between reducing hospitalization costs and increasing PEP costs, with a maximum reduction in incremental cost for a program that offers PEP to individuals aged 50 years or older.
Health impact of antibody therapy
The scientists assumed a situation where antibody therapy was applied to 50% of unvaccinated contacts aged 50 years or above. This corresponded to 28,187 individuals with an antibody treatment. The health impact analysis revealed that the antibody cocktail treatment coverage of 50% resulted in the prevention of 1,813 symptomatic disease, 526 hospitalizations, and 83 deaths.
Expanding the analysis to household contacts aged 20 years or above, they estimated that the antibody therapy could prevent more than 5,000 symptomatic COVID-19, 768 hospitalizations, and 93 deaths. In contrast, the analysis covering household contacts aged 80 years or above resulted in a reduction of 104 symptomatic disease, 67 hospitalizations, and 24 deaths. Notably, a corresponding 50% reduction in all tested clinical outcomes was observed by increasing the therapy coverage to75%.
A variation in the health impact of antibody therapy was observed between ethnic groups, with non-Hispanic Black individuals exhibiting the highest benefit and non-Hispanic White individuals showing the lowest benefit.
Cost-effectiveness of antibody therapy
The scientists estimated that without antibody therapy, the cost of hospitalization due to secondary SARS-CoV-2 infection acquired through household transmission would be 150 million dollars. As observed in further analysis, this cost could be reduced to 147 million dollars by providing antibody therapy to 50% of unvaccinated household contacts aged ≥50 or ≥80 years. In contrast, by considering household contacts aged 20 years or above for antibody therapy, they estimated a total health system cost of 193 million dollars, which is 43 million dollars higher than without antibody therapy.
Study significance
The study findings reveal that the clinical outcomes of COVID-19 as well as treatment costs, could be improved by implementing monoclonal antibody therapies as post-exposure prophylaxis.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources