Immunocompromised patients (ICPs), particularly those with hematologic malignancies and lung cancer, are at a higher risk for severe coronavirus disease 2019 (COVID-19) outcomes as compared to the non-compromised population. Some of these severe outcomes can include intensive care unit admission, invasive ventilation, and death.
The Pfizer-BioNTech BNT162b2 messenger ribonucleic acid (mRNA) vaccine clinical trials showed that the vaccine has 95% efficacy in preventing symptomatic laboratory-confirmed COVID-19. However, the trials excluded ICPs, as their immune response to vaccination cannot be standardized.
Nonetheless, the Israel Ministry of Health has approved vaccination of patients on immunosuppressive therapy or biological response modifiers associated with any malignancy, those having undergone solid organ or stem cell transplantation or splenectomy, as well as patients with human immunodeficiency virus (HIV) or primary immune deficiency.
The strong correlation between vaccine efficacy, the production of protective antibodies that emerged from human vaccine studies, and significant immune response, including receptor binding domain immunoglobulin G (RBD-IgG) and severe acute respiratory coronavirus 2 (SARS-CoV-2) neutralizing titers in sera following the first dose, and more so after the second dose, prompted the evaluation of vaccine efficiency in subpopulations at risk.
In a recent study, Israeli researchers evaluate the antibody response in ICPs with diverse underlying diseases and assess correlates of antibody-mediated immunity following vaccination with the Pfizer-BioNTech vaccine.
About the study
A prospective cohort study was conducted at Sheba Medical Center, Israel, between January and April 2020, in 1,274 participants who received the vaccine, including 1,002 ICPs and 272 immunocompetent healthcare workers (HCWs).
A total of 1,002 selected ICPs had various active malignancies, solid organ transplants (SOTs), allogeneic hematopoietic stem cell transplants (HSCTs), HIV, chronic lymphocytic leukemia and non-Hodgkin's lymphoma (CLL/NHL), multiple myeloma, anti-CD-38 and autologous bone marrow transplantation, and myelodysplastic syndrome (MDS). All patients were treated with some form of immunomodulatory/immunosuppressive drug.
One common drug in most regimens was some form of glucocorticosteroid. Patients receiving anti CD20 monoclonal antibodies during the six months prior to vaccination and patients with acute graft vs. host disease (GVHD) were excluded, as were patients hospitalized for induction therapy for acute leukemia, lymphoma, or transplantation. Patients with a positive SARS-CoV-2 polymerase chain reaction (PCR) test before or after the first vaccination and during the first week after the second vaccination were also excluded.
All participants were vaccinated 21 days apart, with both doses of the Pfizer-BioNTech BNT162b2 mRNA vaccine. Antibodies were measured two-four weeks after vaccination by SARS-CoV-2 anti–RBD-IgG antibody and pseudo-virus neutralization assays.
Serological analysis of samples from participants was tested with an enzyme-linked immunosorbent assay (ELISA) to detect IgG antibodies against the RBD-SARS-CoV-2. Sera not capable of reducing viral replication by 50% at a 1:8 dilution or below were considered non-neutralizing. All positive RBD-IgG samples were tested for neutralizing antibodies.
Study findings
Statistical analysis showed the highest response rate among HIV patients with 154/156 (98.7%) developing antibodies, with a geometric mean (GMT) of 5.14. Patients with solid malignancies also showed a remarkable response, wherein 75/90 (83.3%) developed antibodies, with a GMT of 3.17.
Other groups also showed considerable responses, albeit with lower NA titers. Those with multiple myeloma showed a response in 79.7%, those with HSCT 74.8%, liver transplantation 69.4%, MDS 60.5%, CLL/NHL 51.0%, kidney transplantation 45.0%, and heart transplantation with only 18.8%. For the control group, 269/272 (98.9%) developed antibodies with a GMT of 5.98.
Following the second dose, HSCT and HIV RBD-IgG positive patients developed high neutralizing antibody titers with GMT values of 653.8 and 467.6, respectively. In contrast, heart and kidney transplant patients developed low neutralizing antibody titers with GMT values of 53.8 and 89.5, respectively. The GMT for neutralizing antibodies in the control group was 474.
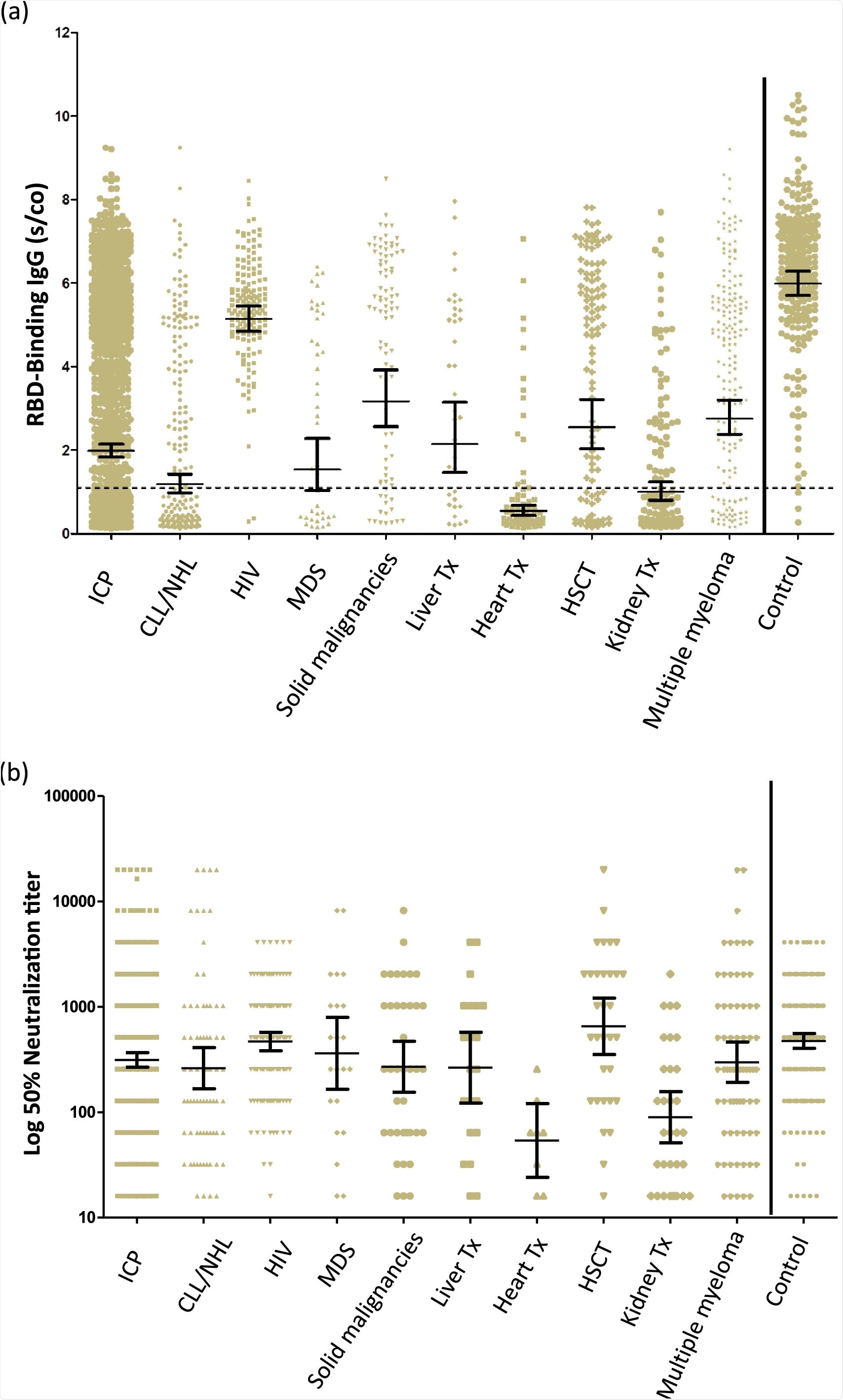 Quantitation of IgG following the second dose of the BNT162b2 vaccine in immunocompromised patients and immunocompetent health care workers. (A) RBD-IgG levels. (B) Neutralizing antibodies above the cutoff. The dotted black line indicates the limit level of positive antibodies. The short black line indicates GMT and 95% CI.
Quantitation of IgG following the second dose of the BNT162b2 vaccine in immunocompromised patients and immunocompetent health care workers. (A) RBD-IgG levels. (B) Neutralizing antibodies above the cutoff. The dotted black line indicates the limit level of positive antibodies. The short black line indicates GMT and 95% CI.
Implications
Overall antibody response to the BNT162b2 mRNA vaccine was highly variable among different immunosuppressed patients. Thus, individual recommendations need to be followed for different classes of immunosuppressed patients.
Despite these findings, it was seen that younger patients, particularly those with HIV infection and solid malignancies being treated with immunochemotherapy, those with multiple myeloma, HSCT recipients six months post-transplant without GVHD, liver transplant patients and other transplant patients not receiving antimetabolite maintenance immunosuppression, were more likely to develop antibody responses. As a result, vaccination is highly recommended in these populations.
In contrast, older patients, particularly those who had previously received heart and kidney transplants, were less likely to develop an antibody response and should be warned to follow strict infection control measures, particularly vaccination of all other household members.
The current study sheds light on vaccination strategies to be followed for the most vulnerable population. Vaccinating as many people as possible is currently the only way to prevent the further spread of SARS-CoV-2.