Several studies have focused on post-infection sequelae in survivors of severe SARS-CoV-2 infection that lead to coronavirus disease (COVID-19). However, not many have tried to understand the long-term health consequences of survivors of mild or asymptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections.
Patients who had experienced mild disease had elevated levels of C-reactive protein 1–3 months after the onset of symptoms and showed changes in circulating T-cell phenotype and function that were absent 6–9 months after symptom onset.
Expression of adherence and chemokine receptors, which indicate altered migratory capacity, and markers of monocyte activation were also higher at 1–3 months post-infection in survivors of mild SARS-CoV-2 infection. However, these parameters were no longer elevated in these individuals at 6–9 months post-infection.
Surprisingly, T-cells activated by polyclonal stimulation were significantly higher in individuals who had recovered from mild SARS-CoV-2 infection than those who recovered from other respiratory infections. Thus, the observations of this study reveal prolonged immune activation as well as systemic inflammation lasting for at least 3 months after a mild or asymptomatic SARS-CoV-2 infection.
COVID-19 causes more significant immune dysregulation than that caused by other respiratory pathogens
Although it is known that severe infections may lead to hospitalization and long-term health consequences, there is little awareness about the lasting respiratory, cardiovascular, and neurologic consequences after a mild SARS-CoV-2 infection. The range of long-term health issues and the immune pathology involving multiple organs after a SARS-CoV-2 infection shows that COVID-19 results in a greater degree of immune dysregulation than that observed after infection by other respiratory pathogens.
It is worth noting that the participants in this study had only mild symptoms and did not experience “long-COVID.” Most of their symptoms resolved within 2–4 weeks. However, it is clear from the results that even participants with mild COVID-19 symptoms had elevated immune activation and inflammation for at least 1–3 months after their infection resolved. Increased systemic inflammation (e.g., CRP) and phenotypic and functional changes to monocytes and T-cells demonstrate that inflammatory responses to mild COVID-19 disease are more complicated than expected.
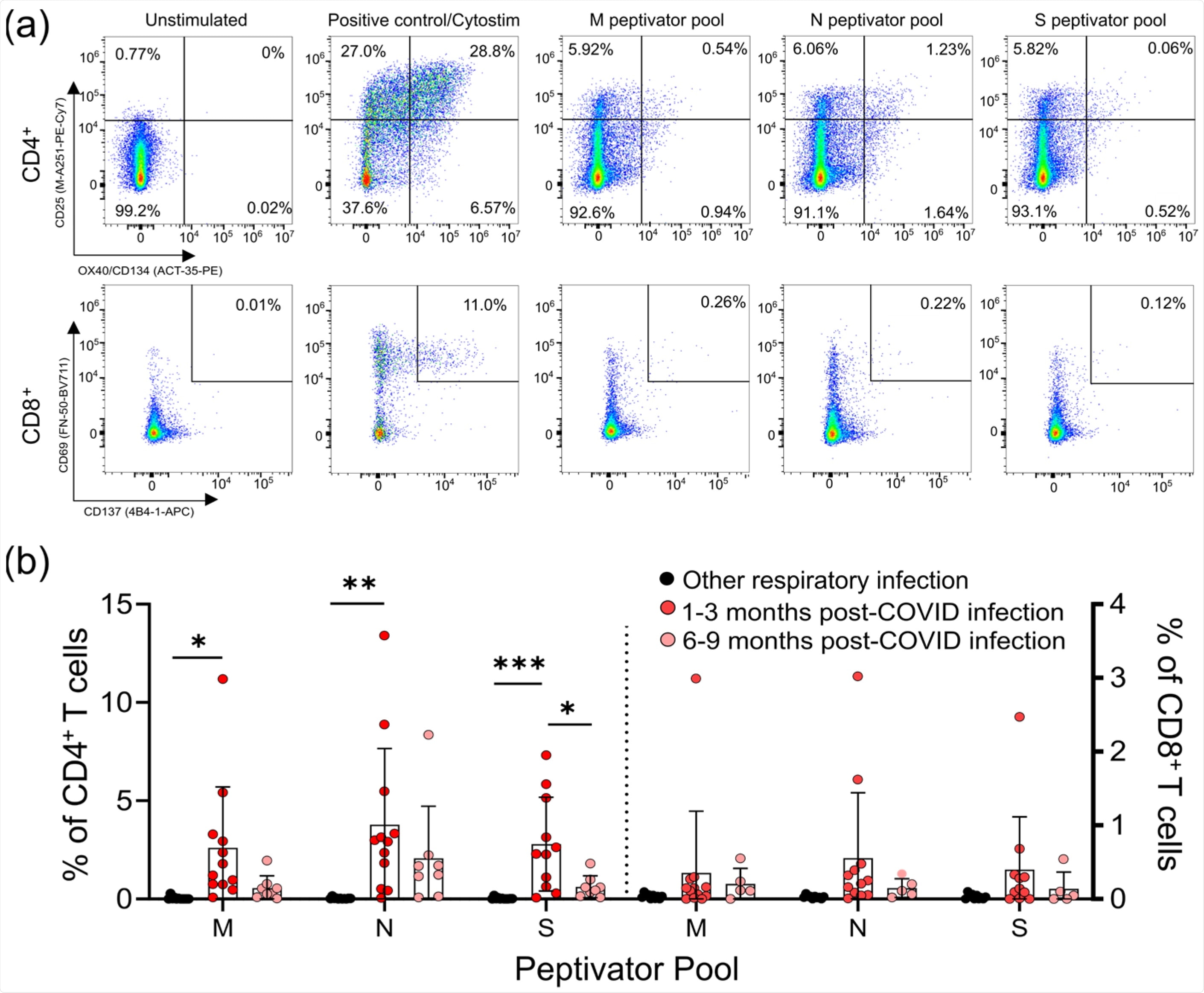
CD4+ and CD8+ T-cell responses to the M, N, and S peptide pools after mild SARS-CoV-2 infection. (a) The number of SARS-CoV-2-specific T-cells is measured as a percent of CD4+ T-cells expressing both CD25 and OX40, or CD8+ T-cells expressing both CD69 and CD137, after activation with the S, M, or N peptide pools 1–3 months and 6–9 months after infection. The polyclonal activator Cytostim is used as a positive control. (b) All COVID-19 seropositive participants had an increase in CD25+OX40+CD4+ T-cells in response to at least one of the M, N, or S antigens 1–3 months after mild COVID-19 infection, compared to seronegative individuals recovered from other mild respiratory infections. Each participant is indicated by a single data point: other respiratory infection n = 11; 1–3 months post COVID-19 infection n = 11; 6–9 months post COVID-19 infection n = 8. Multiple group comparisons were tested using Welch’s One-Way ANOVA and the Games–Howell post-hoc test; bars represent the mean ± standard deviation. * p < 0.05; ** p < 0.01; *** p < 0.001.
The amount of memory CD4+ and CD8+ T-cells specific for SARS-CoV-2 S, M, and N proteins observed in the study were consistent with those seen in convalescent patients who had recovered from severe COVID-19. This implies that COVID-19 disease severity is not directly proportional to SARS-CoV-2 memory T-cell responses. SARS-CoV-2 reactive T-cells were detected in 5 survivors of mild respiratory symptoms with no positive diagnostic PCR test or seropositivity for anti-SARS-CoV-2 antibodies.
The observations of temporary increases in central memory CD4+ T-cells and terminally differentiated CD8+ T-cells are similar to the T-cell responses to acute viral infection. They agree with previous studies showing lasting memory responses in survivors of mild SARS-CoV-2 infections. However, elevated regulatory T-cells are usually associated with minimizing pathology in the acute phase of respiratory viral infections. While severe SARS-CoV-2 infections are triggered by the development of autoantibodies that contribute to disease severity, mild SARS-CoV-2 infections are linked to autoimmune inflammatory syndromes such as arthritis or vasculitis after the primary infection is resolved.
Findings reveal sustained immune activation post symptom resolution in mild or asymptomatic SARS-CoV-2 infections
According to the authors, this study has identified crucial differences in immune responses to SARS-CoV-2 infection and other respiratory infections. One limitation, however, is that the type(s) of non-COVID-19 respiratory infections were not determined in the study. Also, this was not a longitudinal study, so the comparison between the 1–3-month and 6–9-month post-infection groups was made using different individuals.
“It will be interesting to compare our data on immune responses generated by primary infection to future studies examining post-infection immune activation in vaccinated individuals with mild breakthrough infections.”
Overall, the findings offer evidence for mild or even asymptomatic SARS-CoV-2 infections leading to sustained immune activation post symptom resolution, which does not occur in response to other mild respiratory infections. It remains to be seen if this immune activation is more prominent in patients with serious infections or long-COVID.
Journal reference:
- Kennedy, A.E.; Cook, L.; Breznik, J.A.; Cowbrough, B.; Wallace, J.G.; Huynh, A.; Smith, J.W.; Son, K.; Stacey, H.; Ang, J.; McGeer, A.; Coleman, B.L.; Larché, M.; Larché, M.; Hambly, N.; Nair, P.; Ask, K.; Miller, M.S.; Bramson, J.; Levings, M.K.; Nazy, I.; Svenningsen, S.; Mukherjee, M.; Bowdish, D.M.E. Lasting Changes to Circulating Leukocytes in People with Mild SARS-CoV-2 Infections. Viruses 2021, 13, 2239. https://doi.org/10.3390/v13112239, https://www.mdpi.com/1999-4915/13/11/2239/htm