
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The emergence of new SARS-CoV-2 variants of concern (VOCs) remains a threat two years since the COVID-19 pandemic started. Although preventive COVID-19 vaccines have been developed and administered to billions of individuals worldwide, there is a pressing need to develop novel targeted immunotherapies for COVID-19 and other inflammatory diseases in vulnerable and critically ill patients.
About the study
In the present study, the researchers assessed the immune responses in the lungs of COVID-19-infected patients to develop tailored immunotherapeutic approaches to mitigate disease progression. The blood and tissue samples of healthy controls and 583 COVID-19 patients admitted to the Mount Sinai Hospital in New York were collected from the Mount Sinai COVID-19 Biobank, New York City. The cohorts were longitudinally followed from March 2020 through December 2020.
Depending upon how many days after hospitalization the samples were collected, timepoint numbers were assigned to the patients’ peripheral blood mononuclear cells (PBMC) and serum samples. Timepoint 1 (T1) samples were collected at an average of about 15 days of self-reported post-symptom onset (PSO).
An additional sample was collected from critically ill patients hospitalized for more than two weeks, which was seven days later at T13. According to the clinical criteria designated by Mount Sinai Hospital, severity scoring was assigned to each patient sample.
Study findings
The results demonstrate that COVID-19 patients with moderate disease had higher levels of circulating dendritic cells (DC), cytokines associated with antigen-presenting cell (APC) function, and cytotoxic and effector T-cells. Furthermore, this same cohort of COVID-19 patients exhibited low levels of cytokines from monocyte and neutrophil activation, hyper inflammation, and mucosal damage.
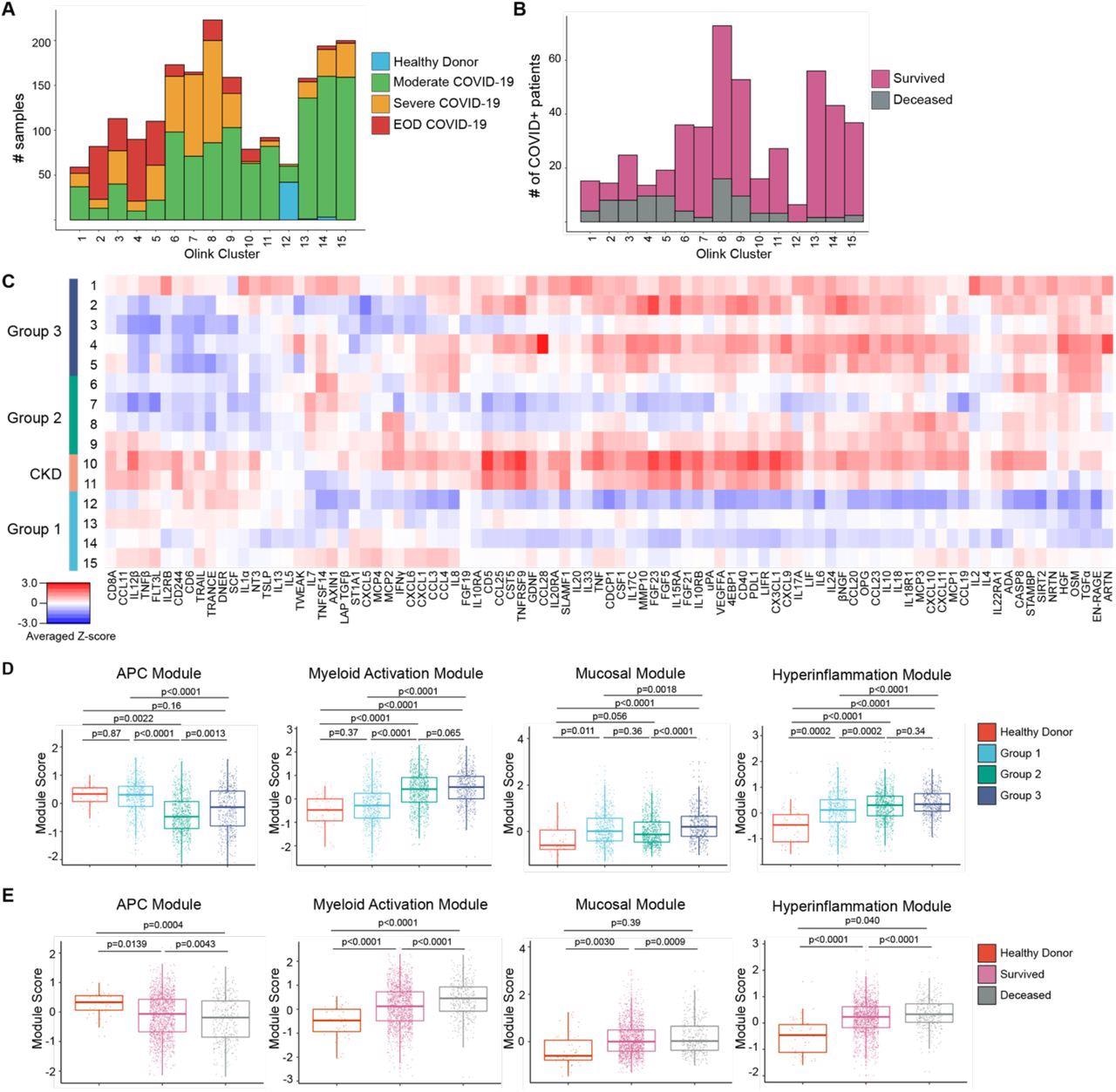
Proteomic characterization of COVID-19 serum reveals distinct immune patterns associated with disease severity and clinical outcome. (A) Histogram of patient samples across Olink clusters denoted by clinical severity classification. (B) Histogram of first available patient samples across Olink clusters denoted by patient projected clinical outcome. (C) Averaged z-scored heatmap of Olink inflammation panel analytes across Olink clusters. Olink clusters were grouped based on clinical severity, projected outcome, and comorbidity distribution. (D) Boxplots showing Olink module score comparisons of all Olink samples by Olink group. (E) Boxplots showing Olink module score comparisons of all Olink samples by final clinical outcome. For box plots, each dot represents a patient sample; center line, median; box limits, 25th and 75th percentile; whiskers, 1.5x interquartile range (IQR). Statistical significance (D-E) determined by 2-way ANOVA with Tukey’s Multiple Comparisons correction. Adjusted p-values shown.
In contrast, severely ill COVID-19 patients showed a reduction in cytotoxic and effector T-cells, DCs, and cytokines associated with APC function. Furthermore, these patients also exhibited high levels of immature inflammatory monocytes producing S100A12, cytokines associated with monocyte and neutrophil activation, hyper inflammation, and mucosal damage.
The AMs isolated from COVID-19-positive patients expressed higher levels of inflammatory cytokines and lower levels of human leukocyte antigen (HLA) class I/II genes as compared to the patients who were negative for COVID-19. Thus, COVID-19-positive patients had low levels of AM and altered antigen (Ag) presentation function.
The AMs isolated from deceased COVID-19 patients were found to be more inflammatory and had high levels of monocyte and neutrophil attracting chemokines as compared to AMs from surviving COVID-19-positive patients.
Elderly patients with low levels of AMs and high levels of inflammatory porcine monocyte-derived macrophages (MoMΦ) were susceptible to more severe COVID-19.
A significant loss of AM-derived receptors for advanced glycation end products (RAGE), such as S100A8, S100A9, and S100A12 ligands, were noted in COVID-19-positive patients. Additionally, lung autopsies of deceased COVID-19 patients demonstrated a shift in the production of RAGE ligands to MoMΦ, infiltrating monocytes, and granulocyte-like cells in the lung interstitium.
Conclusions
The current study presents the systemic and lung high dimensional immunophenotyping data of one of the largest single-centered COVID-19 cohorts to date collected during the peak of the COVID-19 pandemic in New York City.
A significant dysregulation in myeloid cells, as well as the numbers and functionality of AMs, was observed in severe COVID-19 patients. COVID-19 patients with moderate disease severity had a stronger Ag presentation and effector T-cell signature, whereas COVID-19 patients with severe illness were associated with an altered Ag presentation and the replacement of AMs by infiltrating monocytes.
Taken together, the results demonstrate AM depletion-associated recruitment of immature inflammatory myeloid cells from the periphery and tissue damage in SARS-CoV-2 infected patients. Thus, the driving factors of COVID-19 severity include defective Ag presentation by altered AMs and low levels of DC, together with the mobilization of inflammatory monocytes.
Both SARS-CoV-2 infection and a reduction in granulocyte-macrophage colony-stimulating factor (GM-CSF) in the alveolar milieu can lead to the depletion and alteration of the AM pool. Apart from the phagocytic ability of AM, these cells are also involved in lung homeostasis, tissue repair, and the resolution of inflammation.
Overall, the study findings emphasize the importance of early detection and restoration of AM pools during SARS-CoV-2 infection. The maintenance of AM numbers in COVID-19 and other inflammatory diseases might be a valid treatment strategy that could protect airway integrity and initiate early innate and adaptive immune responses while restricting the proliferation and mobilization of immature inflammatory myeloid cells from the periphery.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Chen, S. T., Park, M. D., Del Valle, D. M., et al. (2022). Shift of lung macrophage composition is associated with COVID-19 disease severity and recovery. bioRxiv. doi:10.1101/2022.01.11.475918. https://www.biorxiv.org/content/10.1101/2022.01.11.475918v1
- Peer reviewed and published scientific report.
Chen, Steven T., Matthew D. Park, Diane Marie Del Valle, Mark Buckup, Alexandra Tabachnikova, Ryan C. Thompson, Nicole W. Simons, et al. 2022. “A Shift in Lung Macrophage Composition Is Associated with COVID-19 Severity and Recovery.” Science Translational Medicine 14 (662). https://doi.org/10.1126/scitranslmed.abn5168. https://www.science.org/doi/10.1126/scitranslmed.abn5168.