
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Experimental studies with human pathogens require strict ethical scrutiny, assurance that any potential risks to study participants are minimized and managed, as well as highly regulated and controlled settings. These types of studies have enabled the exact longitudinal measurement of SARS-CoV-2 kinetics, immune responses, viral transmission dynamics, and assessment of the duration of viral shedding post-inoculation with a fixed viral dose.
Experimental studies also deliver unparalleled information that can inform clinical policies and the refinement of infection control measures, thus enabling the rapid evaluation of vaccines, therapeutics, and diagnostics.
About the study
In the present study, researchers enrolled 36 healthy volunteers between the ages of 18 and 29 years who provided written informed consent. All 36 participants were seronegative at the screening by Quotient MosaiQ antibody microarray test and had no history of SARS-CoV-2 vaccination or infection. As two participants turned exhibited seroconversion before inoculation, only 34 participants were screened during the study.
All participants were inoculated with a low inoculum dose of 10 median tissue culture infectious dose (TCID50) of D614G-containing pre-alpha wild-type SARS-CoV-2 intranasally. After the inoculation, 18 study participants developed polymerase chain reaction (PCR)-confirmed infection.
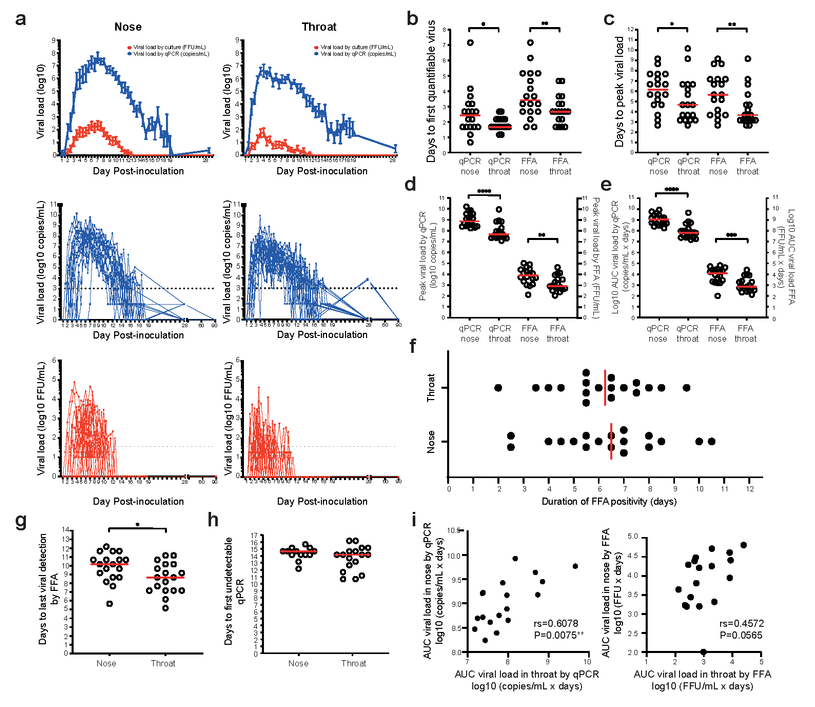
Viral shedding after a short incubation period peaks rapidly following the human SARS-CoV-2 challenge. Healthy adult volunteers were challenged intranasally with SARS-CoV-2. In the 18 infected individuals, (a) VL in twice-daily nose and throat swab samples was measured by qPCR (blue) and focus-forming assay (FFA, red). Results are expressed as mean +/- S.E.M. Dotted lines represent the lower limit of quantification. (b) Median time to the first quantifiable virus (c) peak VL are shown in red. (d) Peak and (e) cumulative (AUC) VL by qPCR and FFA in the nose and throat are compared. Wilcoxon matched-pairs signed-rank tests are used to test significance. (f) Total duration of viral detection by FFA in the nose and throat are shown. Medians are shown in red. (g) Median time to the last viral detection by FFA post-inoculation is shown in red. (h) Median time to the first undetectable VL by qPCR in the individuals who became undetectable while in quarantine is shown in red. (i) AUC VL by qPCR and FFA are correlated in nose vs. throat. Spearman’s r and P values are shown. *P<0.05, **P<0.01, ***P<0.0001, ****P<0.0001.
Study findings
A robust viral replication was observed in 53% (18/34) of seronegative participants. Within two to four days of incubation, viral loads (VLs) escalated rapidly, peaking at high levels and continuing for over a week. Despite high VLs, the symptoms that were reported in 89% of infected individuals were consistently mild-to-moderate and predominantly confined to the upper respiratory tract.
SARS-CoV-2 was detected in the throat of study participants. However, for an average of 10 days post-inoculation (pi), a viable virus could be detected in nasal swabs.
Although common, anosmia/dysosmia resolved in most participants within 90 days without treatment. In individuals showing residual smell disturbance, their sense of smell steadily improved during the follow-up period, as observed in community cases. Radiological assessments showed no evidence of pulmonary disease in infected participants.
Previous studies with non-human primates have not provided conclusive information on optimal SARS-CoV-2 inoculum dose estimates. Even a 10-fold lower dose of 10 TCID50 met the 50-70% target infection rate, thereby suggesting that this model could recapitulate higher exposure than naturally acquired infection events.
Despite the relatively small sample size, there were only limited deviations among infected participants, and longitudinal analysis permitted several conclusions of public health importance.
Detailed viral kinetics showed that some individuals shed culturable virus at 12 days pi, up to 10 days after symptom onset. On average, the viable virus was still detectable 10 days pi. Therefore, these data support the isolation periods of 10 days post-symptom onset advocated in many guidelines to minimize onward transmission.
The observed high levels of asymptomatic/symptomatic VL also highlight the positive impact of routine asymptomatic testing programs that diagnose SARS-CoV-2 infection in the community. Such testing programs allow for the timely implementation of self-isolation that could interrupt community transmission.
A recent re-analysis of cross-sectional lateral flow assay (LFA) validation data also suggested that sensitivity for the infectious virus may be higher than 80%. The longitudinal LFA data following the SARS-CoV-2 challenge also predicted culturable viruses, aside from the very earliest time points. In addition, LFA also reliably predicted the disappearance of viable viruses and reinforced 'test to release' strategies, to shorten the period of self-isolation.
Conclusions
While rare adverse events associated with SARS-CoV-2 infection are detected in large-scale studies, the results of the current study provided first-in-human data revealing that human challenge with SARS-CoV-2 caused no unexpected consequences.
The unique controlled nature of the study model enabled vigorous identification of host factors that conferred protection in those individuals who resisted infection. Future challenge studies should test previously infected and vaccinated volunteers with escalating inoculum doses of SARS-CoV-2 variants to understand the interplay between virus and host factors that influence clinical outcomes.
Taken together, experimental studies like the one conducted by these authors will generate efficacy data of vaccines, antivirals, and diagnostics during their clinical development. The current study data help in circumventing some of the uncertainties on studies that require ongoing community transmission.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Killingley, B., Mann A., Kalinova, M., et al. (2022). Safety, tolerability, and viral kinetics during SARS-CoV-2 human challenge. Nature Portfolio Journal. doi:10.21203/rs.3.rs-1121993/v1.
- Peer reviewed and published scientific report.
Killingley, B., Mann, A. J., Kalinova, M., Boyers, A., Goonawardane, N., Zhou, J., Lindsell, K., Hare, S. S., Brown, J., Frise, R., Smith, E., Hopkins, C., Noulin, N., Löndt, B., Wilkinson, T., Harden, S., McShane, H., Baillet, M., Gilbert, A., & Jacobs, M. (2022). Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nature Medicine. https://doi.org/10.1038/s41591-022-01780-9. https://www.nature.com/articles/s41591-022-01780-9.