With a total of 59 mutations in its genome, 36 of which reside in its S region, Omicron remarkably escapes neutralization by infection- and vaccine-induced antibodies. Thus, it is of keen interest to determine to what extent booster doses of coronavirus disease 2019 (COVID-19) vaccines will compensate for this effect to provide a durable protective antibody response and whether T cells, the other arm of the adaptive immunity, can augment protection against Omicron.
Study: T-cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all individuals
About the study
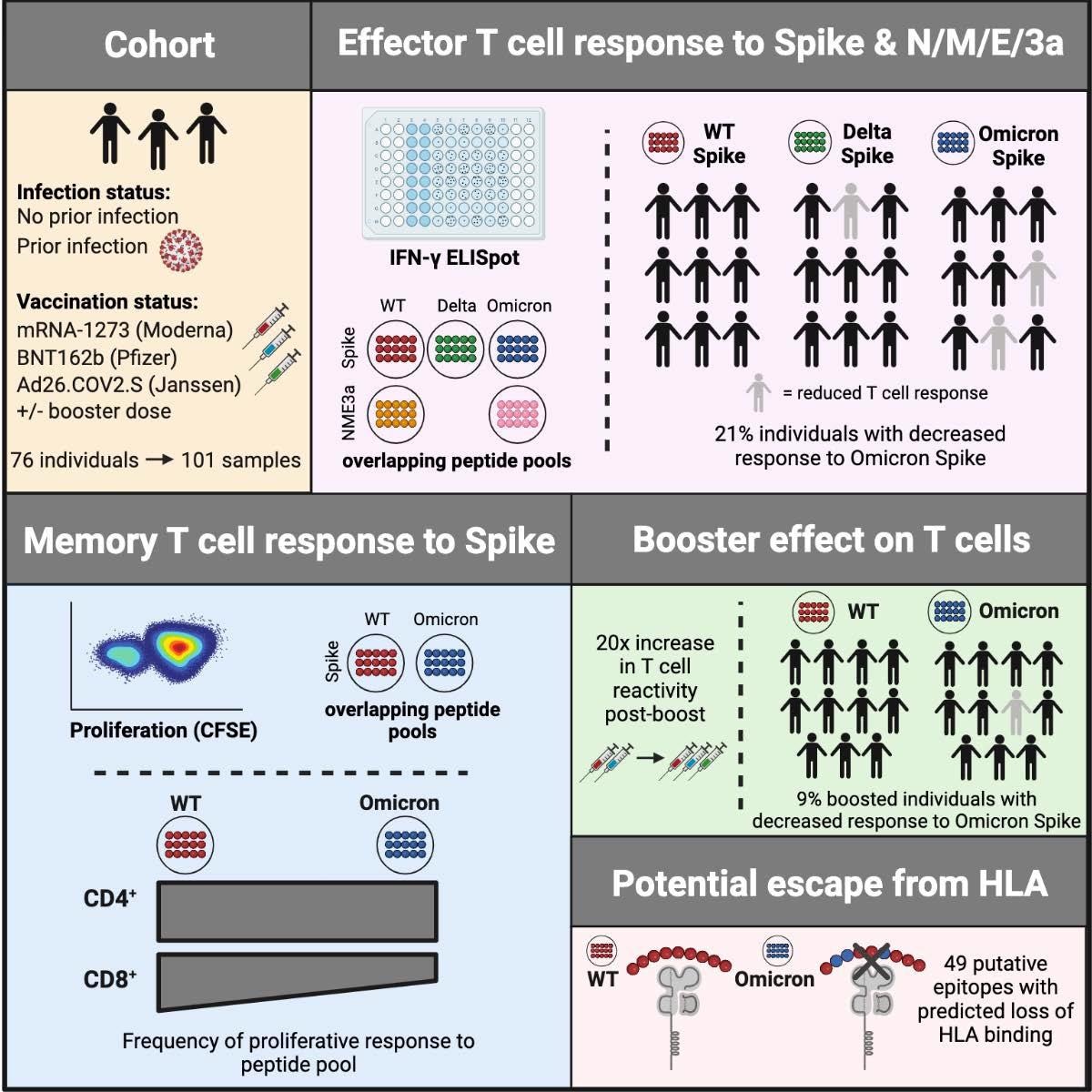
In the present study, researchers studied anti-SARS-CoV-2 T-cell responses in 76 ambulatory adult volunteers in Chelsea, Massachusetts. The subjects were sampled at three-time points – before vaccination, after primary series vaccination, and after receiving additional booster doses.
The researchers studied 101 samples from 76 donors, of which 64% were females. The median age of the study participants was 45 years, and their sera samples were collected on an average 220 days after primary series vaccination or ten days after receiving booster doses.
The researchers sampled both previously SARS-CoV-2-infected and uninfected individuals from both the subsets, including 11 and 10 unvaccinated, 12 and 24 vaccinated (sample taken after primary vaccination), and 13 and 31 vaccinated (sample taken after booster doses) samples, respectively.
The researchers used multivariate regression to analyze the host, vaccine, and SARS-CoV-2 variant variables that affect T-cell responses.
Study findings
The primary multivariate analysis of T-cell reactivity showed that aggregate effector T-cell responses towards Omicron S and structural proteins did not vary by SARS-CoV-2 variant. Additional booster vaccine doses enhanced these T cell responses, but age, sex, and primary vaccine series did not impact the magnitude of these responses.
Further, upon evaluating individual-level responses, the authors noted that a subset of vaccinated individuals and those with prior SARS-CoV-2 infection (~21%) displayed a substantially reduced T-cell reactivity (>50%) against Omicron S but not the Delta variant.
Furthermore, CD8+ T-cell proliferation in response to Omicron S was substantially different than S-specific CD4+ and CD8+ memory T-cell responses, most likely due to escape from human leukocyte antigen (HLA) binding.
Overall, these findings showed that the T-cell responses to Omicron were mostly preserved but with reduced reactivity in some but not all previously SARS-CoV-2-infected and vaccinated individuals. Intriguingly, although booster doses increased antibody and effector T-cell responses, many individuals who were unable to neutralize Omicron pseudotyped virus had measurable T-cell responses against Omicron S prior to receipt of a booster dose.
A majority of amino acids (97.2%) in Omicron spike (S) are preserved in comparison to wild-type (WT) SARS-CoV-2 strain. Moreover, computational HLA-peptide binding affinity assessments have shown that variations in Omicron S affect amino acid residues critical for HLA binding that mediate escape from HLA-restricted T-cell responses induced by prior infection and vaccination.
In the current study, 10 participants showed reduced effector or memory T-cell responses expressing between three to six HLA class I alleles. These HLA alleles are allegedly affected by Omicron S mutations due to a loss in HLA-binding, further supporting the notion that HLA-binding is critical to mount a sustained T-cell response against SARS-CoV-2. These observations should be validated using additional experimental data obtained via specific epitope mapping. Further studies analyzing larger cohorts could also aid in the identification of specific HLA class I alleles that provide disparate susceptibility to viral epitope escape.
Conclusions
The study findings have important implications for Omicron-induced immune responses that could prove very useful in developing second-generation COVID-19 vaccines. Interestingly, most Omicron epitope sequences are conserved and retain binding to HLA-I, and T-cell reactivity to Omicron substantially increases after booster vaccination.
The authors observed that non-spike structural and accessory proteins (nucleocapsid, membrane, enveloped, and open reading frame 3A, NC/M/E/3A) harbored a reduced number of mutations relative to S, hence could serve as potential targets for second-generation COVID-19 vaccines.
Future vaccination strategies that will collectively target conserved, SARS-CoV-2 variant resistant sites to induce robust memory and effector T cell responses alongside antibody responses may therefore yield durable T cell immunity and broad protection against future SARS-CoV-2 variants.
Journal reference:
- T-cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all individuals, Vivek Naranbhai, Anusha Nathan,Clarety Kaseke, Cristhian Berrios, Ashok Khatri, Shawn Choi, Matthew A. Getz, Rhoda Tano-Menka, Onosereme Ofoman, Alton Gayton, Fernando Senjobe, Zezhou Zhao, Kerri J. St Denis, Evan C. Lam, Mary Carrington, Wilfredo F. Garcia-Beltran, Alejandro B. Balazs, Bruce D. Walker, A. John Iafrate, Gaurav D. Gaiha, J., Cell, February 02, 2022 doi: https://doi.org/10.1016/j.cell.2022.01.029, https://www.cell.com/cell/fulltext/S0092-8674(22)00140-4