Anosmia is a recognized symptom in coronavirus disease 2019 (COVID-19), though it is not present in many patients. A new study published on the preprint server medRxiv* discusses the potential utility of using paired ampules of n-butanol to screen for COVID-19 and detect those at greater risk for severe illness.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Introduction
The loss of function in the nasal olfactory epithelium has been well-documented to indicate the potential presence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Notably, older people generally show a decline in olfactory acuity, while also being at higher risk for severe COVID-19.
The presence of anosmia in COVID-19 generally correlates with less severe disease, conversely, with affected patients typically being younger in age. The exception is when the loss of smell is prolonged. The mechanism of anosmia is thought to be the rapid swelling of the olfactory mucosa causing the olfactory cleft to be blocked, thereby preventing odorant molecules from entering it to stimulate the olfactory neurons.
Sudden, unexplained onset of anosmia likely is an indicator of the intensity of the initial inflammatory immune response to viral infection.”
While patients are asked about anosmia, the frequency of detection is correlated better with objective tests. These tests are independent of cultural sensitivities to certain odors, do not rely on familiarity, and require the patient to be able to speak the language used to describe the odorant.
Scratch-and-sniff tests are being used to identify a particular odorant among many; however, graded olfactory stimuli are more useful to provide a direct assessment of olfactory sensitivity. Although several reliable and sensitive tests have been developed, these often require more time to be completed, and their reuse may not be practical in a pandemic situation.
The current study discusses the results of using a disposable test for odorant detection that is both inexpensive and designed for instant results. To this end, the current test used n-butanol at two concentrations of 0.06% and 3.2%, the latter of which was increased to 0.32% later on in the study.
The first test was designed to screen individuals who were naturally hyposmic in the population. The data here were compared with data from the UR Primary Care Central Respiratory Clinic that was established to screen patients with COVID-19-like symptoms during the early days of the pandemic. The average viral positivity at this time was about 9%; however, at this clinic, it was three times greater at 31%.
Once the early surge subsided, the test was continued for preoperative screening of all patients at a single center.
Study findings
Viral infection was linked to anosmia, with the odds being three times higher in the presence of SARS-CoV-2. Patients with a positive SARS-CoV-2 test result were two times more likely to experience anosmia as compared to those who were negative.
Though increasing age is linked to reduced olfactory sensation, with COVID-19 positivity, the opposite trend was seen. More specifically, with a median age of 55 years, older patients tended to have lower risk of altered smell. Furthermore, a higher risk of severe illness and death following hospitalization was observed in patients without anosmia.
As compared to the frequency of dysosmia as recorded by the National Health and Nutrition Examination Survey (NHANES) study, the researchers here found that patients over 40 years of age were associated with a 65% higher incidence of anosmia as compared to the expected rate, with COVID-19 positivity being associated with an increased incidence of anosmia by four times. The risk of anosmia in patients with SARS-CoV-2 infection was thus 2.4 times higher, thus confirming the aforementioned findings.
The likelihood of a positive odorant detection test was higher when the first concentration was very low at 0.06% as compared to the 0.32% concentration, which is likely because of the repeated attempts to smell the odorant at the earlier concentration. Despite this factor, a failure to detect the higher concentration indicates anosmia or severe hyposmia.
Using these two concentrations, the scientists also reported that the age of the patient influences the smell sensitivity to the lower concentration of 0.32%. For instance, anosmia and hyposmia occur in 1.9% as compared to 20.6% of those aged 40-49 years, respectively, as determined by failure to smell 3.2% and 0.32% n-butanol, respectively. In comparison, NHANES anosmia and hyposmia statistics are 3.7% and 0.3%, respectively.
In COVID-19 olfactory dysfunction, the patient first becomes unable to smell lower concentrations, and then anosmia sets in, with recovery occurring in the opposite direction.
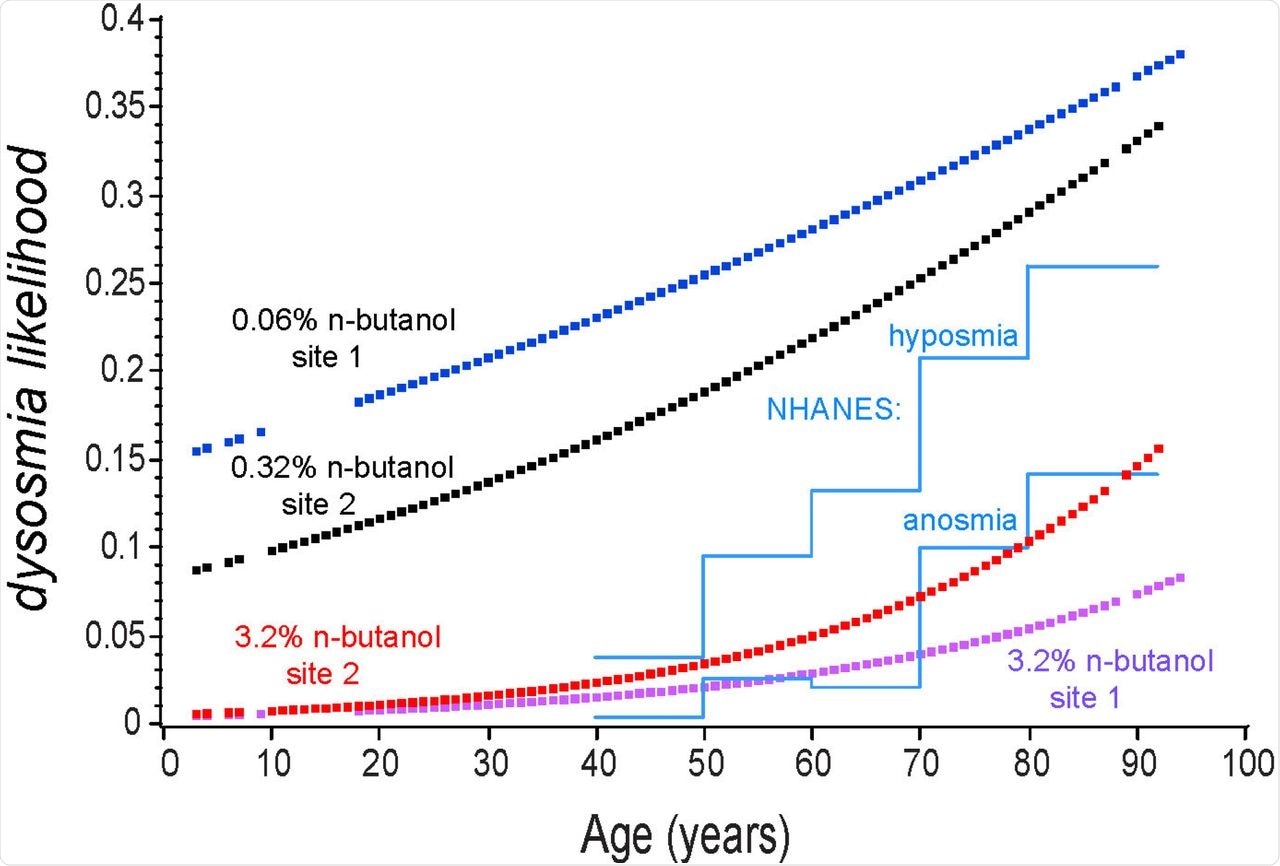
Note that the two low concentrations were more sensitive to olfactory impairment than the NHANEs criteria, but the NHANEs data was in reasonable agreement with the high concentration odorant test. The likelihood of detecting the high concentration odorant was greater at the site where the first test stimulus was more difficult to detect, suggesting repeated attempts to smell a very low concentration stimulus resulted in a greater likelihood of detecting the second and much higher concentration odorant. Blue: 0.06% n-butanol in water at preoperative site 1; Black: 0.32% n-butanol at site 2; Red: 3.2% n-butanol at site 2; Purple: 3.2 % at site 1; Blue lines: NHANES(1) estimates
Implications
We suggest that any patient, regardless of age, who presents with a sudden onset of dysosmia, which cannot be otherwise explained, should be immediately tested for COVID-19 infection.”
The study findings show that changes in smell could be reliably detected using this combination of two odorants, with expected changes as age advances.
Thus, the current approach could be used as a rapid screening method to identify patients who are likely to be infected, while allowing for immediate and repeated testing in a highly cost-effective manner. This will also allow for the early detection of upsurges in the prevalence of COVID-19 indicating an incipient outbreak and patient responses to non-pharmaceutical interventions.
With mass manufacture and deployment, as well as proper reporting systems, such tests could add immensely to available epidemiologic knowledge of the pandemic.
Use in screening and health care contexts could identify those COVID-19 patients at greater risk of severe disease and prompt consideration of immediate medical interventions. If used broadly in conjunction with rapid screening (antigen) tests, viral positivity in conjunction with normal olfaction would enable more rapid recognition and medical intervention for those patients at risk of severe disease and a poorer prognosis.”

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.