A new study assesses the neutralization of three Omicron sublineages by BNT162b2-vaccinated sera collected one month after the third vaccine dose. A preprint version of the study is available on the bioRxiv* server while the article undergoes peer review.
 Image Credit: creativeneko / Shutterstock
Image Credit: creativeneko / Shutterstock
Omicron variant sublineages

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant is the fifth variant of concern (VOC) to date, with the BA.1, BA.2, and BA.3 sublineages. BA.1 was first identified in South Africa in November 2021, after which it became the dominant variant worldwide. BA.2 was then prevalent in many countries. It was more transmissible than BA.1 but did not cause severe coronavirus disease 2019 (COVID-19). BA.3 prevalence has remained low compared to BA.1 and BA.2.
BA.1 causes breakthrough infections and is known to evade immunity conferred by vaccination or prior non-Omicron infection. Possibly due to immune evasion and increased transmissibility, Omicron has replaced the previous Delta variant. These three sublineages harbor different sets of mutations in their spike proteins. Therefore, their susceptibility to neutralization is expected to be different.
BioNTech/Pfizer’s BNT162b2 is an mRNA vaccine that elicits immune responses against the full-length spike protein from the original SARS-CoV-2 Wuhan-Hu-1 isolate. The US Food and Drug Administration has approved the BNT162b2 vaccine for vaccinating people 16 years and above and authorized emergency use for vaccinating 5 to 15-year-old children.
Calculating the neutralizing antibody levels
The BA.1, BA.2, and BA.3 full-length spike proteins with the mNeonGreen (mNG) fluorescent reporter were engineered into USA-WA1/2020, a SARS-CoV-2 strain isolated in January 2020. The insertion of mNG allowed the development of a fluorescent focus reduction neutralization test (FFRNT). A high-throughput FFRNT is a reliable test to measure antibody neutralization.
Diluted serum was mixed with mNG SARS-CoV-2 and added to Vero E6 cells. The serum mNG SARS-CoV-2 was removed after 1 hour of infection. Fluorescent foci were measured and normalized to the non-serum-treated controls to estimate the relative infectivities. The FFRNT50 value was defined as the minimal serum dilution that reduced >50% of fluorescent foci. The levels of neutralizing antibodies in the serum were calculated.
Post-vaccination sera were obtained from participants of Pfizer-BioNTech clinical trials C4591001 and NCT04368728, one month after the third dose of the BNT162b2 vaccine.
Neutralizing antibody levels against Omicron
A previous study has shown that sera after the second dose of the BNT162b2 vaccine did not elicit robust neutralization against Omicron BA.1. Many individuals have received a booster dose of the vaccine. Therefore, post-dose 3 sera were used in this study.
All sera neutralized recombinant viruses. However, the neutralizing antibody levels against BA.1, BA.2, and BA.3 were 3.6-, 4.0-, and 6.4-fold lower than those against the USA-WA1/2020 strain.
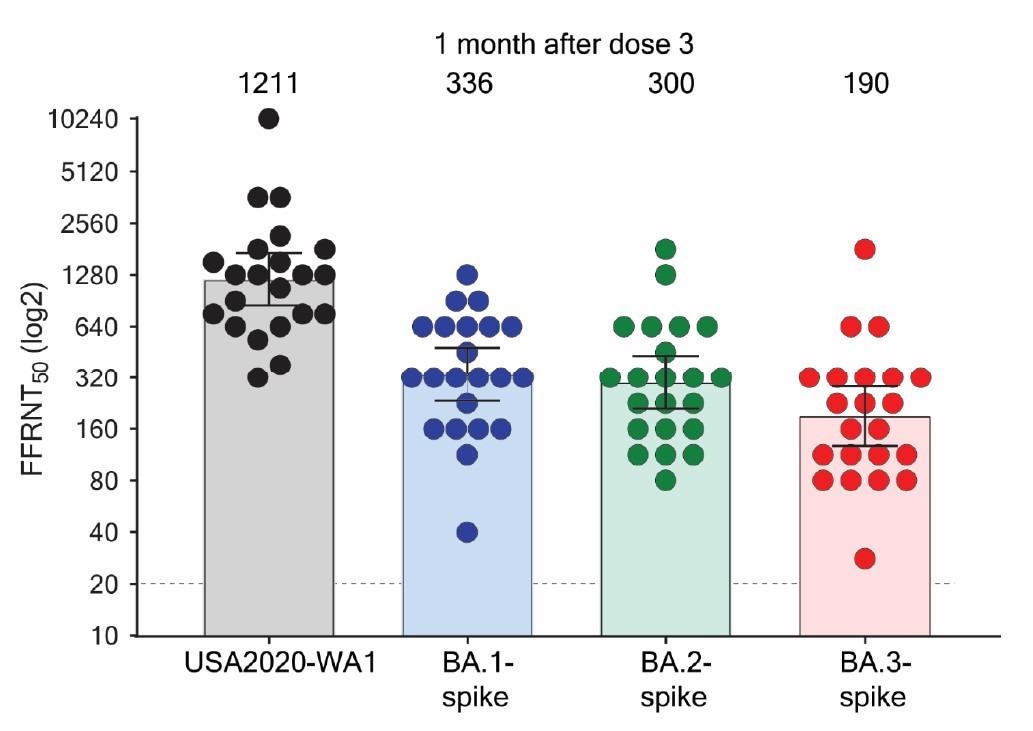
Serum neutralization of Omicron BA.1-, BA.2-, and BA.3-spike mNG SARS-CoV-2s and USA-WA1/2020 after three doses of BNT162b2.
Limitations of the study
This study has assessed the neutralizing antibody levels in sera one month after the third dose. Reducing antibody levels over time or vaccine waning is known to reduce immunity against symptomatic infection. However, studies are underway to assess the durability of neutralizing antibody levels against Omicron sublineages and future variants.
Implications of the study
The BA.1 and BA.2 spike proteins evaded neutralization by the sera at comparable levels. This suggests that the increased prevalence of BA.2 over BA.1 in the population may not be due to a difference in antibody neutralization after vaccination. It is possibly due to other factors like differences in viral replication and transmission or other immune evasion mechanisms.
BA.3 spike protein evaded neutralization by the sera more efficiently than BA.1 and BA.2 spike proteins. The BA.3 sublineage can potentially expand and extend the current surges of the Omicron variants. Thus, BA.3 genomic surveillance should be closely monitored.
Apart from neutralizing antibodies, other immune effectors, including T cells and non-neutralizing antibodies, also contribute to the immune protection against severe outcomes of COVID-19. The majority of T cell-mediated immunity against Omicron spike protein is preserved after vaccination or natural infection. Three doses of the BNT162b2 vaccine have been effective against Omicron infection. However, real-world data has shown waning protection against symptomatic infection by the Omicron variant. The third dose confers immunity against severe COVID-19 until 3 to 6 months post-vaccination. The durability of these immune responses needs to be studied.
These studies along with real-world vaccine effectiveness data will inform the decision to recommend a 4th dose of the vaccine for optimal breadth and duration of protection.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Kurhade C, Zou jing, Xia H, et al. (2022). Neutralization of Omicron BA.1, BA.2, and BA.3 SARS-CoV-2 by 3 doses of BNT162b2 vaccine. bioRxiv. 2022.03.24.485633. doi:10.1101/2022.03.24.485633. https://www.biorxiv.org/content/10.1101/2022.03.24.485633v1
- Peer reviewed and published scientific report.
Kurhade, Chaitanya, Jing Zou, Hongjie Xia, Hui Cai, Qi Yang, Mark Cutler, David Cooper, et al. 2022. “Neutralization of Omicron BA.1, BA.2, and BA.3 SARS-CoV-2 by 3 Doses of BNT162b2 Vaccine.” Nature Communications 13 (1). https://doi.org/10.1038/s41467-022-30681-1. https://www.nature.com/articles/s41467-022-30681-1.