New research shows fat distribution predicts premature cardiovascular ageing more than BMI, revealing why visceral and liver fat harm the heart while lower-body fat protects women before menopause.
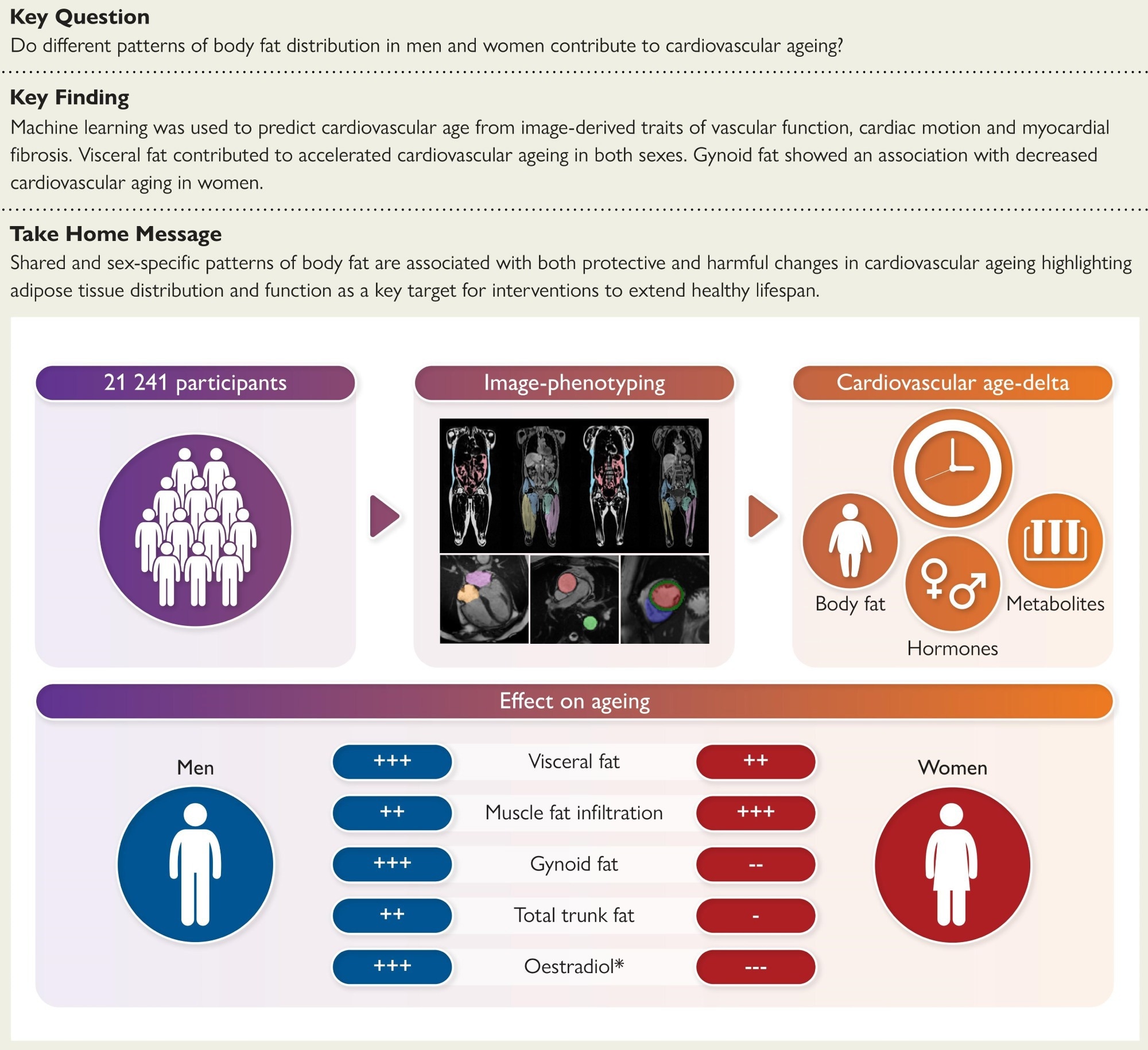
Structured Graphical Abstract: The association of body fat phenotypes and cardiovascular ageing was assessed in 21,241 participants. This showed how shared and sex-specific patterns of body fat are associated with protective and harmful changes in cardiovascular ageing. *Protective effects of oestradiol in pre-menopausal women.
In a recent article in the European Heart Journal, researchers investigated how different patterns of body fat distribution influence cardiovascular aging by gathering data from more than 20,000 individuals.
Their findings indicate that liver fat, muscle fat infiltration, and visceral fat predicted accelerated cardiovascular aging in men and women. Abdominal subcutaneous adipose tissue and android fat predicted higher cardiovascular age-delta in men only, while total abdominal adipose tissue was associated with adverse outcomes in both sexes. Gynoid fat was protective in pre-menopausal women, but in men, it was associated with a higher age-delta.
Background
Obesity is a complex condition marked by excess fat accumulation that harms health. It is increasing worldwide, affecting nearly half of adults. While obesity is commonly measured by body mass index (BMI), people with similar BMI can have very different risks for cardiovascular disease. This occurs largely because fat distribution matters.
Visceral fat, stored deep in the abdomen, is particularly harmful. It promotes vascular problems, inflammation, and metabolic dysfunction. Obesity also accelerates cardiovascular ageing. This process signals declining physiological resilience. It is linked to inflammation, genetic and metabolic factors, and tissue dysfunction.
Sex differences further complicate this picture. Women generally store more fat in the lower body, while men accumulate more visceral fat, which increases cardiovascular risk. Female sex hormones, especially before menopause, may offer protection by influencing metabolism and fat distribution. However, it remains unclear how these sex-specific fat patterns affect cardiovascular ageing.
About the study
This study analyzed data from over 21,000 UK Biobank participants aged 40–69, including those with existing cardiovascular disease, to capture the lifetime impact of fat distribution on cardiovascular ageing.
Cardiovascular age was predicted using a pre-trained machine learning model based on 126 imaging traits of cardiac structure, function, vascular dynamics, and myocardial tissue composition. The difference between predicted and actual age was calculated as the ‘cardiovascular age-delta.’
Whole-body and regional fat distribution were measured with magnetic resonance imaging (MRI) and dual-energy X-ray absorptiometry (DXA). This included assessments of gynoid, android, liver, muscle-infiltrated, visceral, and subcutaneous fat.
Cardiac MRI provided detailed measures such as ventricular volumes, ejection fraction, aortic distensibility, myocardial strain, and fibrosis. All imaging data underwent automated segmentation and quality control.
Associations between fat phenotypes and cardiovascular age-delta were tested with multivariable linear regression, stratified by sex. Additional analyses examined BMI, physical activity (via questionnaire-based metabolic equivalent scores), and blood biomarkers (lipids, sex hormones, and metabolites).
To explore causality, Mendelian randomization was conducted using genetic instruments from genome-wide association studies of fat depots and cardiovascular ageing. Finally, cardiovascular events such as heart attack, stroke, and heart failure were identified from health records, and their relationship with cardiovascular age-delta was assessed using Cox proportional hazards models.
Key findings
Researchers noted clear sex-related differences in fat distribution. Women generally had more abdominal subcutaneous, muscle-infiltrated, and gynoid fat, while men carried higher levels of visceral, android, and total body fat.
Age-related patterns also diverged; visceral fat increased more steeply in men, while subcutaneous fat declined slightly in both sexes.
Across the cohort, visceral fat, liver fat, muscle fat infiltration, and total abdominal fat were consistently associated with accelerated cardiovascular ageing in both sexes. However, sex-specific effects emerged.
In men, android and abdominal subcutaneous fat were linked to higher cardiovascular age-delta, while in women, gynoid, trunk, and whole-body fat were protective, especially before menopause. BMI was a weaker predictor than direct measures of fat distribution.
Physical activity partially reduced but did not eliminate visceral fat’s adverse impact on cardiovascular ageing.
Diabetes amplified the harmful impact of visceral and other fat depots, though metformin users showed a somewhat reduced effect.
Genetic analyses supported a protective role of gluteofemoral (gynoid-type) fat, while visceral and abdominal subcutaneous fat showed non-significant but harmful directions of effect.
Biomarker analyses revealed that higher low-density lipoprotein (LDL), total cholesterol, and apolipoprotein B were associated with faster cardiovascular ageing, while high-density lipoprotein (HDL) and favorable lipid metabolites were protective.
Sex hormones also played a role: oestradiol was protective in pre-menopausal women but adverse in men, while free testosterone was linked to slower ageing in both sexes.
Cardiovascular events were ascertained from health records and modeled with Cox methods; atrial fibrillation and type 2 diabetes were associated with higher cardiovascular age-delta, whereas MACE and all-cause mortality were not significant in this cohort.
Conclusions
This study demonstrates that obesity contributes to premature cardiovascular ageing, but fat distribution, rather than overall body mass, is the critical determinant. Visceral and liver fat, along with muscle fat infiltration, accelerated cardiovascular ageing in both sexes, while gynoid fat was protective in women, likely influenced by oestradiol before menopause.
These findings suggest that hormonal regulation and fat depot biology jointly shape sex differences in aging and emphasize the limitations of BMI as a risk measure, highlighting imaging-based fat assessment as a more precise tool.
Potential interventions include pharmacological strategies that reduce visceral and liver fat while mitigating inflammation and cellular senescence, for example, GLP-1 receptor agonists, with emerging roles suggested for SGLT2 inhibitors and other pathways, alongside lifestyle measures like diet and exercise.
Limitations include under-representation of older adults, limited ancestral diversity, and a cross-sectional design, which prevents tracking changes over time. Additional limitations include a lack of external validation at an equivalent scale, potential MR biases from sample overlap and pleiotropy, and unmeasured factors such as VO₂max and detailed diet. Overall, the study identifies adipose tissue distribution as a key modifiable factor in cardiovascular ageing and a promising target for extending healthspan.
Journal reference:
- Sex-specific body fat distribution predicts cardiovascular ageing. Losev, V., Lu, C., Tahasildar, S., Senevirathne, D.S., Inglese, P., Bai, W., King, A.P., Shah, M., de Marvao, A., O’Regan, D.P. European Heart Journal (2025). DOI: 10.1093/eurheartj/ehaf553, https://academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehaf553/8237967