Background
Filoviruses, including Marburgvirus, Ebola virus, and Cuevavirus, are enveloped, single-stranded, negative-sense ribonucleic acid (RNA) viruses that cause deadly hemorrhagic fever in humans and non-human primates.
The viral genome of filoviruses encodes eight proteins, including matrix protein and RNA polymerase. The only viral protein that is displayed on the surface of mature virions is the glycoprotein (GP), which mediates viral entry into host cells.
In some filoviruses, including the Ebola virus and Cuevavirus, the GP gene encodes a trimeric surface GP and dimeric soluble GP. The soluble GP is much more abundant than the surface GP and may play a role in immune evasion. Because of its abundancy, soluble GP absorbs the majority of cross-reactive antibodies, leaving only a small fraction of antibodies available to neutralize the surface GP.
The glycoproteins of different filoviruses are antigenically distinct because of virus-specific sequence diversity of up to 70%. Fundamentally, the monomeric GP of filoviruses contains two subunits known as GP1 and GP2 that are connected by a single disulfide bond.
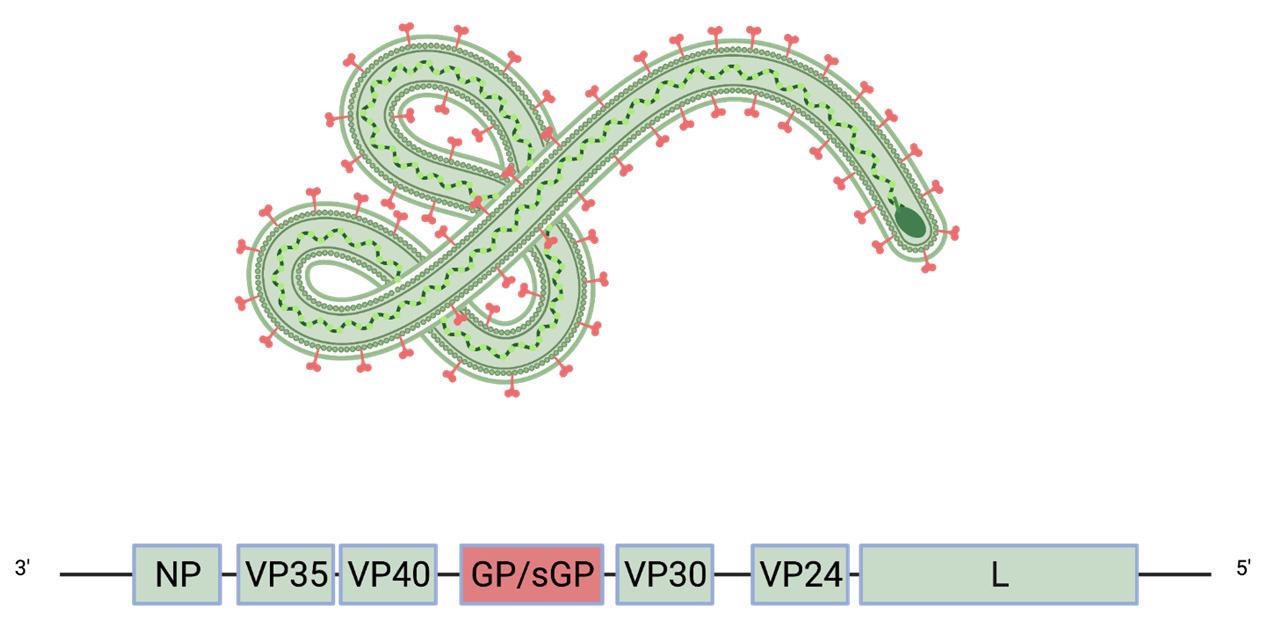
Schematic of the Ebola virus genome and virion. The glycoprotein GP (red) is the only viral protein displayed on the virion surface.
The GP1 subunit contains the receptor-binding site (RBS), glycan cap domain, and heavily glycosylated mucin-like domain (MLD). Comparatively, the GP2 subunit contains host membrane fusion machinery.
Filoviruses enter host cells via endosomal pathways. In the endosome, the glycan cap and mucin-like domain are cleaved from the glycoprotein by endosomal cathepsins. The cleaved glycoprotein binds to the host cell receptor through the receptor binding site, followed by fusion between the viral envelope and the host cell membrane.
Development of antibodies
The surface expression of GP makes it the primary target for developing anti-filovirus antibodies.
Several monoclonal therapeutic antibodies have been identified from immunized animals and human survivors of filovirus infections. However, some early antibodies isolated from human survivors have failed to protect non-human primates from Ebola infection. Subsequently, various non-neutralizing antibodies with protective efficacy were isolated from the hybridomas of immunized mice.
With further advancement, several neutralizing antibodies targeting GP epitopes have been identified. To this end, these antibodies have exhibited high efficacy in ameliorating disease symptoms and improving survival.
A criterion has been set by the Viral Hemorrhagic Fever Immunotherapeutics Consortium (VIC), which indicates that therapeutic monoclonal antibodies against filoviruses should ideally have neutralization and immune effector functions.
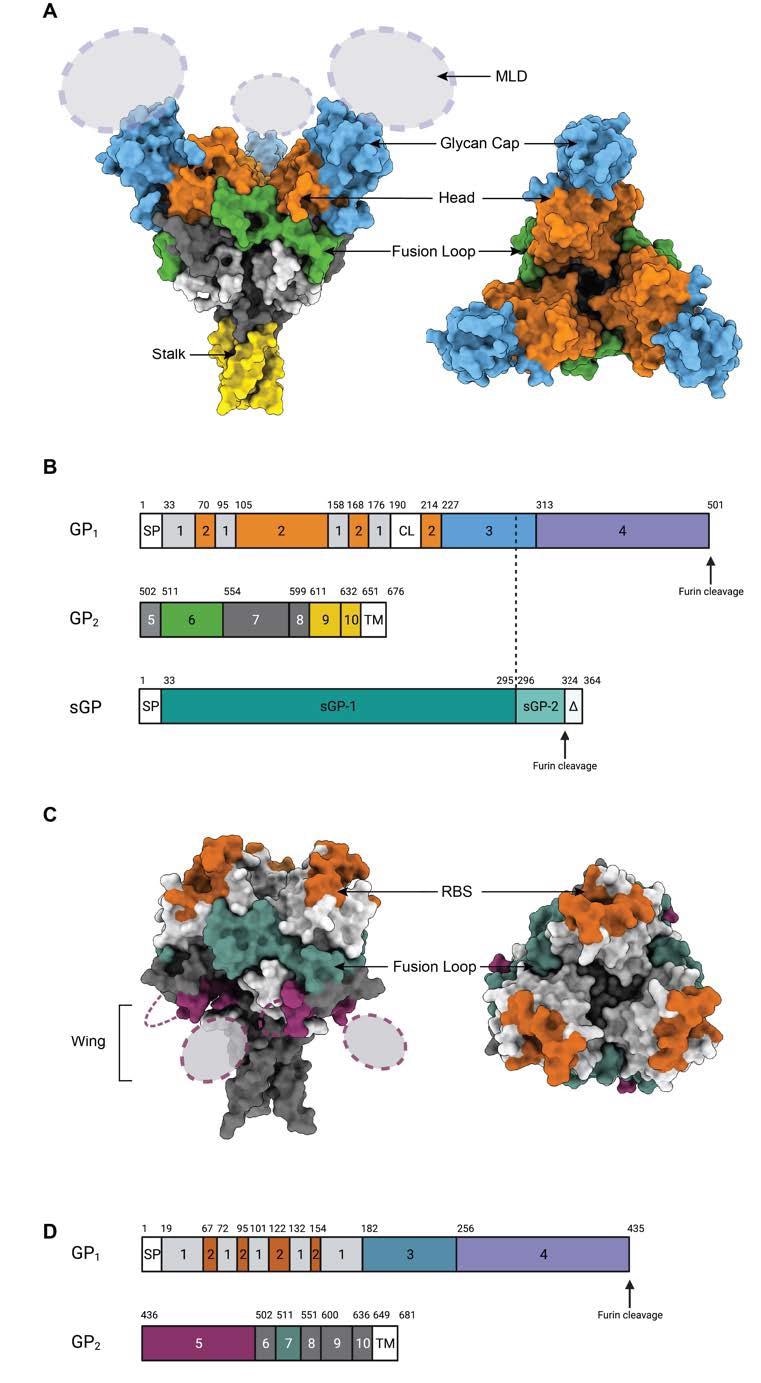
Epitopes on the GP surface. (A) Surface representation of Ebola Virus GP structure (PDB: 5JQ3) colored by domain. Side view and top view of Ebola virus GP are illustrated. (B). Schematic of the EBOV GP sequence. Amino acid numbering is at top, and polypeptide regions that form key domains are numbered in the center of the schematic blocks. 1: portions of the N-terminus of GP1 that form the base, 2: receptor-binding head, 3: glycan cap, 4: mucin-like domain (MLD), 5: GP2 N-terminal peptide; 6: fusion loop, 7: Heptad repeat 1 (HR1), 8 and 9: Heptad repeat 2 (HR2); 9: stalk; and 10: and membrane-proximal external region (MPER), respectively. Other regions include SP: signal peptide, and TM: transmembrane domain. The organization of sGP is illustrated below. The first 295 residues are identical to those in GP1 (labeled sGP-1). Residues 296 through 324 are unique to sGP (labelled sGP-2). The C-terminal sequence, termed delta peptide, is released from sGP by furin cleavage. (C) The surface representation of Marburg Virus GP structure (PDB: 6BP2) colored by domain. Side view and top view of Marburg virus GP are illustrated. (D) Schematic of the MARV GP sequence. Amino acid numbering is at top. 1–2: GP1, with 2 for RBS; 3: glycan cap, 4: MLD, 5: wing; 6: N-terminal loop: 7: fusion loop, 8: HR1, 9: HR2; 10: MPER; SP: signal peptide, and TM: transmembrane domain.
Monoclonal antibodies targeting glycan cap
Antibodies targeting the glycan cap of the Ebola virus generally show cross-reactivity to soluble GP, as the majority of glycan cap residues are present on both surface and soluble GPs. These antibodies are characterized by high levels of immune effector functions and low neutralizing efficacy. Furthermore, these antibodies induce neutralization by blocking cathepsin-mediated cleavage of GP, which is needed for viral entry.
Higher numbers of interactions between anti-glycan cap antibodies and mucin-like domain make the GP trimer unstable. As a result, these antibodies can be synergized with antibodies targeting the fusion loop.
Despite low neutralizing efficacy, anti-glycan cap antibodies are considered good candidates for therapeutic cocktails because of their combined neutralizing and immune effector functions, as well as the ability to synergize with fusion loop targeting antibodies.
Monoclonal antibodies targeting receptor binding site
Antibodies targeting the head epitope in the receptor-binding site exhibit high neutralizing and protective efficacy by blocking receptor binding. Recently, an antibody targeting the apex epitope in the receptor-binding site has been identified, which shows high neutralizing efficacy against the Ebola virus and the Sudan virus. This antibody targets the center of the GP and binds one Fab to one GP trimer.
Further analysis of the apex-targeting antibody reveals that despite lacking neutralization potency, the antibody binds to the recombinant glycoprotein of the Bundibugyo virus. This indicates that the antibody provides protection against Bundibugyo virus infection through Fc-mediated effector functions.
Monoclonal antibodies targeting fusion loop
Because of the high degree of sequence conservation, the internal fusion loop is considered to be the ideal candidate epitope for developing cross-reactive antibodies. The fusion loop region is divided into two parts including the stem/base and loop/paddle. Among anti-fusion loop cross-reactive antibodies, some target the loop region and part of the base region, whereas the remainder target the stem region and other parts of the base region.
Overall, monoclonal antibodies targeting the internal fusion loop region exhibit broad neutralizing potency against a wide range of filoviruses, including the Ebola virus, Sudan virus, and Bundibugyo virus.
Monoclonal antibody cocktails
Recent immunotherapies including cocktails of two or three monoclonal antibodies with different epitopes and functions have shown promising outcomes in treating deadly filovirus infections. In these cocktails, one anti-fusion loop antibody is combined with another antibody targeting the glycan cap or the head/apex region of the receptor-binding site.
These antibody cocktails have shown high efficacy in preventing Ebola virus infections and protecting against post-exposure fatal outcomes.
Journal reference:
- Yu, X. & Saphire, E. O. (2022). Development and Structural Analysis of Antibody Therapeutics for Filoviruses. Pathogens 11(3). doi:10.3390/pathogens11030374.