Primary care delivery has been considerably disrupted due to COVID-19. However, there hasn't been an evaluation of how the SARS-CoV-2 pandemic affected medication prescribing safety. PINCER is an activity program for reducing hazardous prescribing of high-risk medicines in general practices by conducting manual audits on practice subgroups.
 Study: Changes in English medication safety indicators throughout the COVID-19 pandemic: a federated analysis of 57 million patients’ primary care records in situ using OpenSAFELY. Image Credit: Adul10 / Shutterstock
Study: Changes in English medication safety indicators throughout the COVID-19 pandemic: a federated analysis of 57 million patients’ primary care records in situ using OpenSAFELY. Image Credit: Adul10 / Shutterstock

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
About the study
In the present population-based cohort study, researchers assessed the changes in compliance using during the SARS-CoV-2 pandemic using PINCER prescribing safety indicators.
Data from electronic health records (EHR) of 56.8 million national health service (NHS) patients were assessed. The patients were aged above 18 years and registered with general practices. The primary objective was an evaluation of the monthly changes in trends and the variations in between practices to assess the compliance between September 2019 and September 2021. For the analysis, 13 PINCER indicators were used and grouped as follows: (1) medications that could use gastrointestinal (GI) bleeds; (2) cautioned medications; and (3) medications that require blood test monitoring.
Each of the indicators was calculated as a percentage based on a numerator and denominator ratio, where the numerator denoted the number of patients at risk of a potentially hazardous prescribing event. The denominator was the number of patients for whom the PINCER assessment was clinically meaningful. A higher PINCER indicator percentage indicated less safe prescribing of medications.
For every PINCER indicator, mean percentages for Q1 2020 and 2021 were calculated, representative of the pre-COVID-onset and post-COVID-onset periods, respectively, to assess the impact of COVID-19 on the hazardous prescribing. Data were analyzed using the OpenSAFELY platform and the education management information systems (EMIS) or the test productivity pack (TPP) software.
Results
Data of 1,813,058 NHS patients registered across 6,367 practices at risk of a minimum of one hazardous prescribing event were analyzed in this study. During the SARS-CoV-2 pandemic, hazardous prescribing was largely unchanged. Only minor changes were observed in the PINCER indicators.
There were transient delays in blood test monitoring for some medications, especially ACE inhibitors for which monitoring worsened with time, increasing from 5.2% in Q1 2020 to 12% in Q1 2021. However, all PINCER indicators showed significant recovery by September 2021. For ACE inhibitors, the assessment window was much longer (15 months) than for other blood test monitoring indicators (six months for amiodarone and three months for lithium and methotrexate).
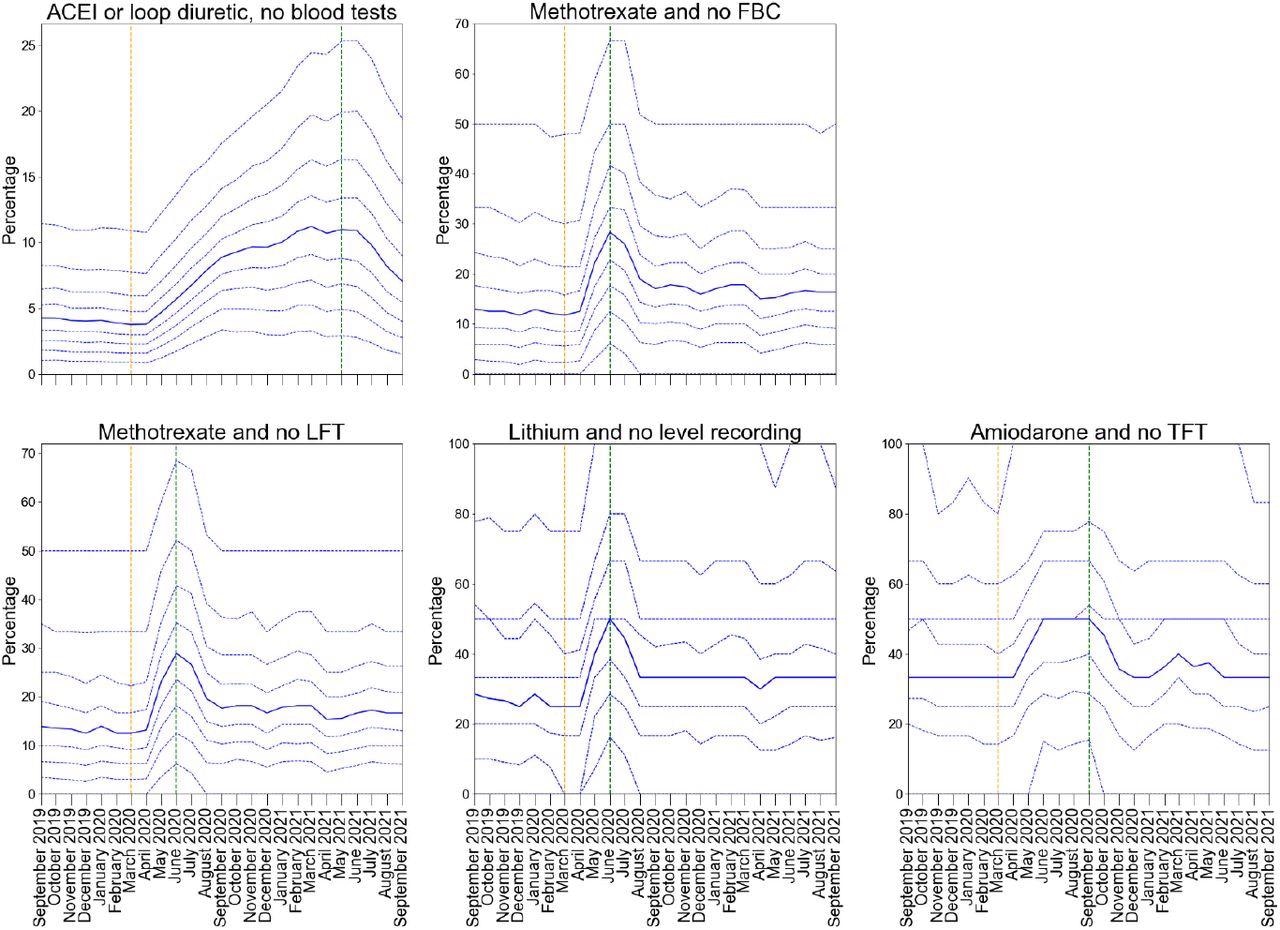
Practice level decile plots for PINCER blood test monitoring indicators. The percentage of patients identified as at risk of potential blood test monitoring as measured by each indicator is reported for the period September 2019 to September 2021 (inclusive). The median percentage is displayed as a thick blue line and deciles are indicated by dashed blue lines. The month of national lockdown in England as a response to the onset of COVID-19 (March 2020) is highlighted with orange dashed vertical line. The monitoring window, as measured from the onset of COVID-19, for each indicator is shown by a green dashed vertical line. All deciles are calculated across 6367 practices (2546 OpenSAFELY-TPP + 3821 OpenSAFELY-EMIS practices).
The percentage of patients identified to be at risk of a potentially hazardous prescribing event ranged between 2.5% (Aspirin & other antiplatelet drugs) and 89.5% (lithium and no level recording). The ratio of hazardous events to patients ranged between 3.8 [non-steroidal anti-inflammatory drugs (NSAID) and PU] and 10.9 (antiplatelet and PU). The percentage of general practices with an event for every indicator ranged between 87% [NSAID and chronic renal failure (CRF)] and 99% (beta-blockers and asthma).
Among the indicators associated with GI bleeding, potentially hazardous prescribing exhibited a declining trend with minimal effects of COVID-19. The mean percentages of NSAID and Warfarin/DOAC were 1.2% and 1.4% in Q1 2020 and Q1 2021, respectively. The percentage of antiplatelet and PU reduced from 4.2% in Q1 2020 to 3.9% in Q1 2021. Likewise, COVID-19 was also found to have minimal effects on compliance for all the PINCER indicators associated with cautioned medications.
Among the indicators associated with blood test monitoring, all indicators exhibited an increase in delayed monitoring post-COVID-19 onset (between May and July 2020). However, the indicators showed substantial recovery between August and September 2020. The mean percentages for Q1 2020 versus Q1 2021 for lithium and no level recording were 31.5% versus 38.4%; for methotrexate and no full blood count (FBC) were 18.6% versus 22.7%; for methotrexate and no liver function tests (LFT) were 19.6% and 23.3%; and for amiodarone and no thyroid function tests (TFT) indicator were 36.2% versus 39.2%, respectively.
Conclusions
In general, the study findings indicate that despite the barriers to primary care delivery during the SARS-CoV-2 pandemic, good performance was maintained across all safety prescribing indicators. Although medication-related blood tests were delayed, all indicators showed substantial recovery by the end of the study period.
Study authors believe it is the most comprehensive assessment of medication prescribing safety during the SARS-CoV-2 pandemic in England, using validated indicators to include nearly all (95%) of the country's population.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
- Preliminary scientific report.
Changes in English medication safety indicators throughout the COVID-19 pandemic: a federated analysis of 57 million patients' primary care records in situ using OpenSAFELY Louis Fisher, Lisa E. M. Hopcroft, Sarah Rodgers, James Barrett, Kerry Oliver, Anthony J. Avery, Dai Evans, Helen Curtis, Richard Croker, Orla Macdonald, Jessica Morley, Amir Mehrkar, Seb Bacon, Simon Davy, Iain Dillingham, David Evans, George Hickman, Peter Inglesby, Caroline E. Morton, Becky Smith, Tom Ward, William Hulme, Amelia Green, Jon Massey, Alex J. Walker, Chris Bates, Jonathan Cockburn, John Parry, Frank Hester, Sam Harper, Shaun O'Hanlon, Alex Eavis, Richard Jarvis, Dima Avramov, Paul Griffiths, Aaron Fowles, Nasreen Parkes, Ben Goldacre, Brian MacKenna medRxiv 2022.05.05.22273234; doi: https://doi.org/10.1101/2022.05.05.22273234, https://www.medrxiv.org/content/10.1101/2022.05.05.22273234v1