Despite the introduction of vaccines, the coronavirus disease 2019 (COVID-19) remains a significant problem around the world, particularly for people with weakened immune systems and those who have not been immunized.
Most COVID-19 patients experience mild to moderate symptoms. However, between 15-20% of patients experience hyper-inflammation caused by excessive cytokine production, a condition known as the "cytokine storm," which can lead to alveolar injury and respiratory failure.
Study: Olverembatinib inhibits SARS-CoV-2. Image Credit: Juan Gaertner / Shutterstock.com
Background
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant was first discovered in late November 2021 in South Africa and has since been linked to rapidly growing case numbers around the world. The Omicron variant consists of over 50 mutations as compared to the ancestral SARS-CoV-2 strain, about 30% of which can be found in the S1 N-terminus domain (NTD).
Stimulating monocytes and peripheral blood mononuclear cells (PBMCs) with the S1 component of the SARS-CoV-2 spike protein promotes overexpression and secretion of an array of inflammatory molecules such as interleukin (IL)-1b, IL-6, and chemokine ligand 7 (CCL7). Furthermore, activation of the S1 NTD is capable of activating monocytes.
In a recent EMBO Molecular Medicine study, researchers determine whether mutations in the Omicron NTD influence its potential to stimulate myeloid cells and enhance inflammatory cytokine release.
Omicron NTD activity
The authors first stimulated pooled PBMCs isolated from healthy donors in various age groups with the Omicron NTD generated from mammalian HEK293 cells. This subsequently led to the release of IL-1, IL-6, and tumor necrosis factor α (TNF-α), thus indicating that the Omicron NTD remains capable of activating myeloid cells and enhancing cytokine release.
The effects of NTD activation on the release of cytokines in PBMCs produced by the Omicron, Delta, and ancestral SARS-CoV-2 strains was also studied. The Omicron NTD was similarly potent in inducing cytokine release.
Study findings
Ponatinib, which is a drug used to treat chronic myelogenous leukemia (CML), was previously discovered to be a powerful inhibitor of S1 protein-mediated cytokine production in PBMCs. As a result, the researchers of the current study were interested in determining whether ponatinib could similarly halt cytokine production mediated by the Omicron variant NTD.
In addition to ponatinib, the researchers also determined the effectiveness of baricitinib, which is a Janus kinase (JAK) inhibitor used to treat active rheumatoid arthritis, and olverembatinib, a clinical-stage multikinase inhibitor that is chemically comparable to ponatinib, in mediating cytokine release caused by the Omicron NTD.
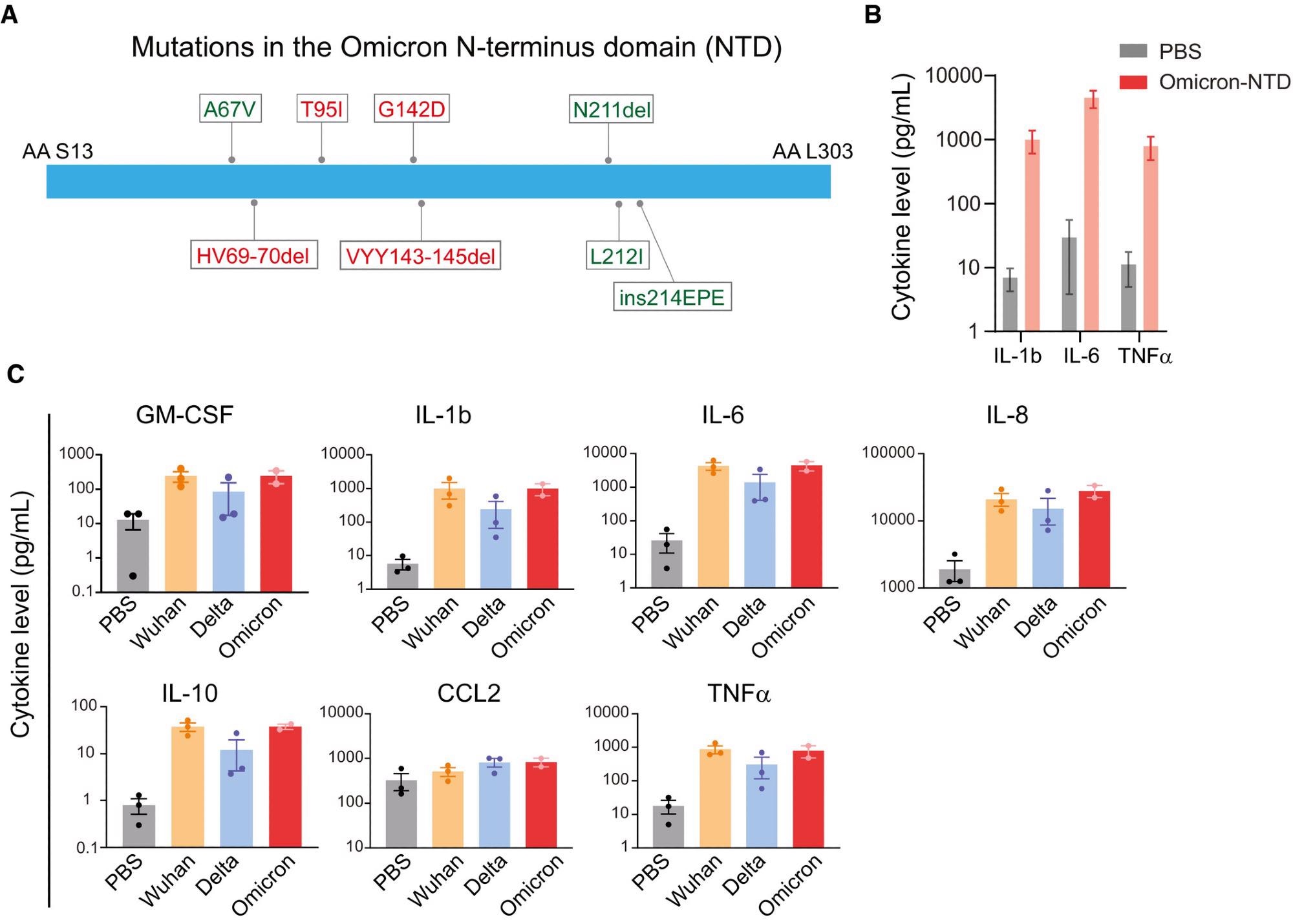 (A) A schematic showing mutation in the Omicron NTD. Unique mutations found in the Omicron variant are shown in green color. (B) Changes in cytokine release in response to the recombinant NTD from Omicron variant. Measurement of cytokine release from healthy donor PBMCs treated with PBS or Omicron NTD at 1 µg/ml for 24 h. Cytokines were measured by Luminex multiplex assay. (C) Comparison of cytokine release from healthy donor PBMCs treated with Wuhan, Delta, or Omicron NTD at 1 µg/ml for 24 h. Cytokine release in the conditioned media was measured by Luminex. Recombinant NTD of different variants were purified from HEK293 cells. Data are shown as the mean of two to three biological replicates. Error bars denote SEM.
(A) A schematic showing mutation in the Omicron NTD. Unique mutations found in the Omicron variant are shown in green color. (B) Changes in cytokine release in response to the recombinant NTD from Omicron variant. Measurement of cytokine release from healthy donor PBMCs treated with PBS or Omicron NTD at 1 µg/ml for 24 h. Cytokines were measured by Luminex multiplex assay. (C) Comparison of cytokine release from healthy donor PBMCs treated with Wuhan, Delta, or Omicron NTD at 1 µg/ml for 24 h. Cytokine release in the conditioned media was measured by Luminex. Recombinant NTD of different variants were purified from HEK293 cells. Data are shown as the mean of two to three biological replicates. Error bars denote SEM.
Even with low nanomolar (nM) concentrations, both ponatinib and olverembatinib suppressed the secretion of IL-1b, IL-6, IL-8, IL-10, TNF-α, granulocyte-macrophage colony stimulating factor (GM-CSF), and CC motif chemokine ligand 2 (CCL2). Comparatively, baricitinib treatment only suppressed the expression of GM-CSF, CCL2, and IL-10.
Olverembatinib therapy resulted in the most significant inhibition of Omicron-NTD-mediated cytokine release. More specifically, the olverembatinib concentration range needed to inhibit 50% of Omicron NTD-mediated cytokine release (EC50) was between 7.7 and 56 nM for all seven cytokines.
The kinase activity profile of olverembatinib revealed that this agent suppresses 11 of the 13 kinases thought to be important for NTD-mediated chemokine and cytokine production. These findings suggest that olverembatinib, a clinical-grade multi-specific kinase inhibitor, inhibits the function of many kinases involved in cytokine signaling, thus reducing the amount of cytokines released by the Omicron NTD.
Olverembatinib responsiveness was also evaluated in PBMCs isolated from nine COVID-19 patients. To this end, Omicron variant-NTD activation significantly increases the production of all five cytokines tested in COVID-19 PBMC conditioned medium.
Notably, a subgroup of cytokines and chemokines had a wide range of responses to Omicron variant-NTD, implying intrinsic patient variability. However, in all COVID-19 PBMCs, treatment with 100 nM olverembatinib completely inhibited the NTD-mediated cytokine storm.
Implications
The current study indicates that drugs like olverembatinib and ponatinib, which target numerous kinases involved in SARS-CoV-2-mediated cytokine production, could be an appealing approach for the treatment of moderate-to-severe COVID-19.
Journal reference:
- Chan, M., Holland, E. C., & Gujral, T. S. (2022). Olverembatinib inhibits SARS-CoV-2. EMBO Molecular Medicine. doi:10.15252/emmm.202215919