Respiratory diseases like obstructive sleep apnea (OSA), asthma, and chronic obstructive pulmonary disease (COPD) continue to contribute to global morbidity and mortality worldwide. In a recent article published in the MDPI journal Microorganisms, researchers review the role of the gut microbiome in these respiratory diseases and how modulating these bacterial species within the body could provide therapeutic benefits to mitigate these conditions.
About the study
An enormous number of microorganisms, collectively called the microbiome, inhabit various parts of the human body, including the gut, skin, and other mucosal environments. The microbiome is critical to the development of the host's innate and adaptive immune systems, whereas the immune system is responsible for maintaining key features of the host-microbe symbiosis.
Previous studies have reported that respiratory conditions like OSA, asthma, and COPD likely arise due to complex interactions between genetic and environmental factors. These same factors often affect the composition of the gut microbiome; therefore, it is important to understand how the gut microbiome contributes to chronic respiratory diseases.
What is the lung-gut axis?
Although the gut and lungs are anatomically distinct, they share a common microbiota that may facilitate their communication and complex pathways.
Numerous factors contribute to alterations of the gut microbiome and similarly have known adverse effects on the development of lung disease. For example, some dietary interventions may impact the respiratory microbiome and, as a result, immunity within this organ system. Insomnia associated with OSA may also influence the gut microbiome by increasing the individual's appetite.
Tobacco smoking, the most common risk factor for COPD, can also alter the gut microbiome. For example, in active smokers, Bacteroidetes are more abundant, while Firmicutes are less prevalent.
Exposure to air pollution, most fine particulate matter (PM2.5), nitrogen oxide, sulfur oxide, ozone, and heavy metals can worsen existing COPD and contribute to its development. Similarly, exposure to certain environmental chemicals may also negatively impact the gut microbiome, ultimately leading to dysbiosis. For example, nitric oxide exposure has been associated with a reduced abundance of Clostridium leptum and Faecalibacterium prausnitzii, as well as increased levels of Dialister genus, Escherichia coli, Enterococcus faecalis, and Proteus mirabilis.
Drugs such as glucocorticoids and antibiotics, commonly used to treat respiratory diseases, have also been shown to precipitate dysbiosis.
The gut microbiome can support immune responses by directly communicating with immune cells. Conversely, the systemic circulation of specific bacterial metabolites such as lipopolysaccharides (LPS) and short-chain fatty acids (SCFA) may lead to airway inflammation, worsening existing inflammatory respiratory conditions.
Asthma
Asthma, which affects over 300 million people worldwide, is characterized by a wide range of symptoms primarily due to chronic hyperinflammation of the airways. Previous research based on the hygiene hypothesis, which states that reduced infection rates throughout the developed world have been accompanied by a rise in allergic and autoimmune diseases, has implicated the gut microbiome in the development of atopic asthma.
In one study, researchers observed that infants with a history of atopy and wheezing had reduced levels of Faecalibacterium, Veillonella, Lachnospira, and Rothia during their first 100 days of life, as well as reduced SCFA levels. Another study on one-year-olds found that the abundance of Veillonella was directly correlated to the development of asthma at five years of age; however, this was only true in children born to asthmatic mothers. Taken together, these reports indicate that alterations in the gut microbiome during the first year of life can directly impact the child's risk of developing asthma.
Although gut dysbiosis may contribute to asthma development, the vast differences in microbiome composition and asthma presentation limit the ability to arrive at firm conclusions on the relationship between these two conditions. Nevertheless, future studies that apply standardized microbiome analyses may better understand gut bacteria's role in the development of asthma.
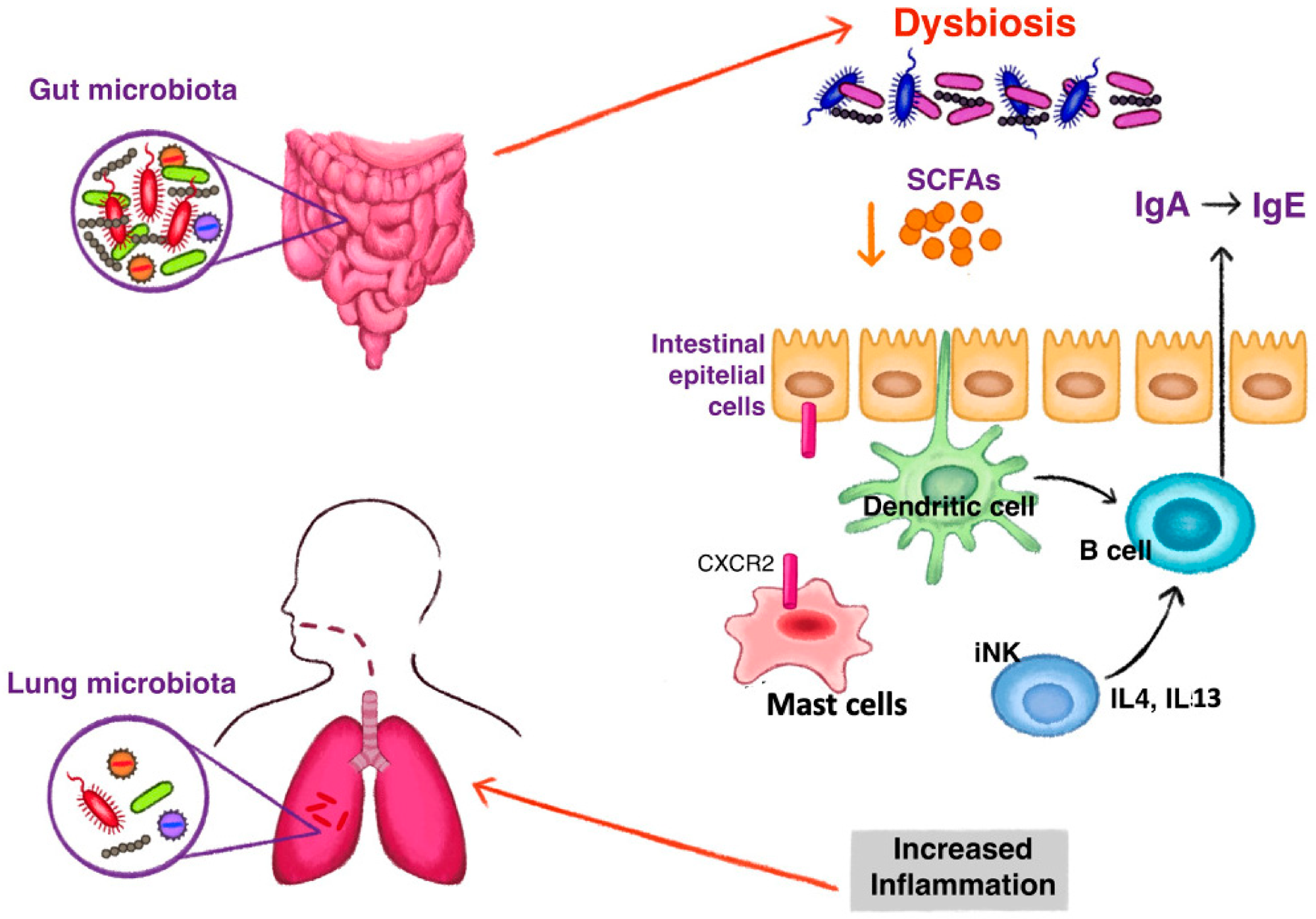 The mechanism of gut dysbiosis leading to the development of type 2 inflammation in asthma. Reduction of short chain fatty acids (SCFAs) induce a class switching of immunoglobulin (Ig) with an increase of fecal IgE acting on dendritic cells. Switching to IgE production is also stimulated by high levels of interleukin (IL) 4 and IL 13, produced by invariant natural killer (iNK) cells under dysbiosis stimuli. Dysbiosis influences the homing of mast cells to the intestine by the expression of CXCR2. Fewer intestinal mast cells and increased blood levels stimulate an inflammatory state observed in asthma.
The mechanism of gut dysbiosis leading to the development of type 2 inflammation in asthma. Reduction of short chain fatty acids (SCFAs) induce a class switching of immunoglobulin (Ig) with an increase of fecal IgE acting on dendritic cells. Switching to IgE production is also stimulated by high levels of interleukin (IL) 4 and IL 13, produced by invariant natural killer (iNK) cells under dysbiosis stimuli. Dysbiosis influences the homing of mast cells to the intestine by the expression of CXCR2. Fewer intestinal mast cells and increased blood levels stimulate an inflammatory state observed in asthma.
Chronic obstructive pulmonary disease (COPD)
COPD is a chronic and progressive respiratory disorder that typically affects the airways, lung parenchyma, and vasculature. Some factors that are directly implicated in the development of COPD, including exposure to harmful chemicals and a history of smoking, may directly impact the gut microbiome composition in these patients.
There remains a lack of large-scale studies investigating the relationship between COPD and the gut microbiome, thus limiting the ability of researchers to truly understand how gut dysbiosis may impact the progression of COPD.
Nevertheless, one study that included 28 COPD patients found an increased abundance of Streptococcus, Rothia, Intestinibacter, and Romboutsia in COPD patients and reduced levels of Bacteroides, Lachnospira, and Roseburia as compared to controls.
Streptococci and Lachnospiraceae abundance in these COPD patients was inversely related to lung function. In another study, an increased abundance of Acinetobacter and Stenotrophomonas were associated with reduced lung function in COPD patients.
Obstructive sleep apnea (OSA)
OSA is a common disorder characterized by the continuous collapse of the upper airways during sleep, leading to altered sleep patterns and frequent periods of hypoxemia. The adverse effects of OSA increase the patient's risk of developing various cardiovascular, metabolic, and neurological diseases.
To date, limited studies have investigated the relationship between OSA and the gut microbiome. In one study on two-year-old children, the Firmicutes/Bacteroides ratio was higher, and reduced microbial diversity was observed among children who snored compared to non-snorers. Similarly, low microbial diversity was observed in a cohort of children between the ages of two and 12 with OSA, in addition to a higher abundance of Proteobacteria in these patients.
How can microbiome-centered treatments improve respiratory conditions?
Several studies have investigated how probiotics, prebiotics, different diets, and fecal microbiota transplantation (FMT) that modulate the gut microbiota can also be used to improve the trajectory of certain respiratory diseases.
Oral supplements
Several in vivo mouse studies have evaluated how incorporating specific dietary components may impact immune responses in the lungs. For example, a high fiber diet that increased circulating SCFA levels protected mice against allergic airway disease, whereas the inoculation of Lactobacillus johnsonii in the guts of mice reduced their Th2 response in the lungs.
Furthermore, supplementation with Bifidobacterium lactis BB-12, docosahexonic acid, as well as vitamins C and E effectively reduced lung inflammation in mice previously exposed to air pollution. Lactobacillus rhamnosus and Bifidobacterium breve probiotic supplementation in mice have also reduced airway inflammation and damage to the alveoli.
The effects of various oral supplements have also been evaluated in numerous human trials. For example, Bifidobacterium longum BB536 supplementation in children between the ages of two and six years old effectively reduced the duration of common upper respiratory tract infections (URTIs) in this population. Similarly, supplementation with Lactobacillus plantarum DR7 for 12 weeks significantly reduced nasal symptoms, the frequency of URTIs, as well as plasma levels of various pro-inflammatory markers, including interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α).
Fecal microbiota transplantation (FMT)
Researchers have also proposed FMT as a novel approach to re-establishing gut flora in patients with certain respiratory diseases. In experimental studies, mice subjected to FMT and subsequently provided a high-fiber diet exhibited an increased abundance of Bacteroidaceae and Lachnospiraceae, which was accompanied by a reduced likelihood of the mice experiencing severe symptoms associated with COPD.
In another study assessing the impact of FMT on LPS-induced lung injury, downregulation of TLR4/NK-kB signaling was observed, along with reduced inflammation and oxidative stress in the lungs of animals with acute lung injury. Similar results were observed in another study, wherein FMT improved the response of germ-free mice following bacterial infection.
Despite these observations, more work is needed to determine the safety of FMT and the benefits associated with this treatment for respiratory diseases.
Journal reference:
- Bikov, A., Dragonieri, S., Csoma, B., et al. (2022). The role of Gut Bacteriome in asthma, chronic obstructive pulmonary disease and obstructive sleep apnoea. Microorganisms. doi:10.3390/microorganisms10122457