The rapid outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in the pandemic of the coronavirus disease 2019 (COVID-19), which has claimed more than 6.7 million lives worldwide. Scientists worldwide have been working at an unprecedented speed to understand varied aspects of SARS-CoV-2, with many studies confirming the development of ‘long COVID’ or persistent post-acute infection syndromes (PASC) in patients previously infected with SARS-CoV-2.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Background
Some of the common symptoms of long COVID include hypogeusia, hyposmia, sleep disorders, and cognitive impairment. One mechanism that has been proposed to contribute to these symptoms include the ability of SARS-CoV-2 to infect multiple types of nerve cells and cause considerable structural changes in the brains of the patients.
Interestingly, transcriptomic profiles, based on post-mortem brain tissues, have established a robust link between cognitive decline in patients with severe SARS-CoV-2 infection and molecular signatures of brain aging. To this end, post-mortem biopsy reports revealed that SARS-CoV-2-infected lungs contained a high level of senescence as compared to uninfected counterparts.
Senescence is a cellular phenotype that influences organismal aging and comorbidities, particularly chronic degenerative conditions. In vivo models of neuropathology and animal studies using physiologically aged mice have revealed that senescent cells promote cognitive decline and neurodegeneration. Nevertheless, the role of senescent cells in COVID-19 pathology, particularly related to human tissue brain aging and in the central nervous system (CNS), remains unclear.
Brain aging has been associated with cognitive decline, which has been attributed to multiple molecular processes including inflammation and cellular senescence. This phenomenon has been established in studies related to normal murine aging and age-related mouse models of neurodegeneration.
Further research is needed to determine whether the endogenous age-related onset of cellular senescence affects brain aging in humans. In addition, it is imperative to understand if the consequence of neurotropic viral infections expedites the onset of cellular senescence in the brain.
Several strategies, including pharmacological interventions, have been adopted to eliminate target senescent cells. At present, several human clinical trials are being conducted to assess the effectiveness of removing senescent cells using senolytic drugs.
Previous studies have demonstrated that an oral administration of dasatinib and quercetin (D+Q) cocktail, or fisetin, affects blood-brain barrier (BBB) permeability, thereby making these agents valuable in assessing the contribution of senescence in the brain.
About the study
A recent study posted on the bioRxiv* preprint server documents the effectiveness of multiple senolytic interventions in removing senescent cells in physiologically aged human pluripotent stem cell-derived brain organoids.
To this end, eight-month-old human brain organoids (BOs) were generated from embryonic stem cells. The newly generated BOs were exposed to two doses of senolytics for one month at two-week intervals. ABT-737, the Bcl-2 inhibitor navitoclax, and D+Q senolytic drug cocktail were tested against the BOs.
Study findings
The exposure of senolytics in physiologically aged BOs exhibited a considerable reduction of senescence-associated β-galactosidase activity1 (SA-β-gal) activity as compared to the control. This finding indicates that all senolytic treatments were able to clear a large number of senescent cells in the treated BOs.
Senescent cells were found to accumulate in the physiologically aged BOs of human origin. Importantly, four weeks (long-term) of intermittent senolytic treatment significantly decreased inflammation and cellular senescence. Here, D+Q treatment triggered antiaging and pro-longevity gene expression in the treated BOs.
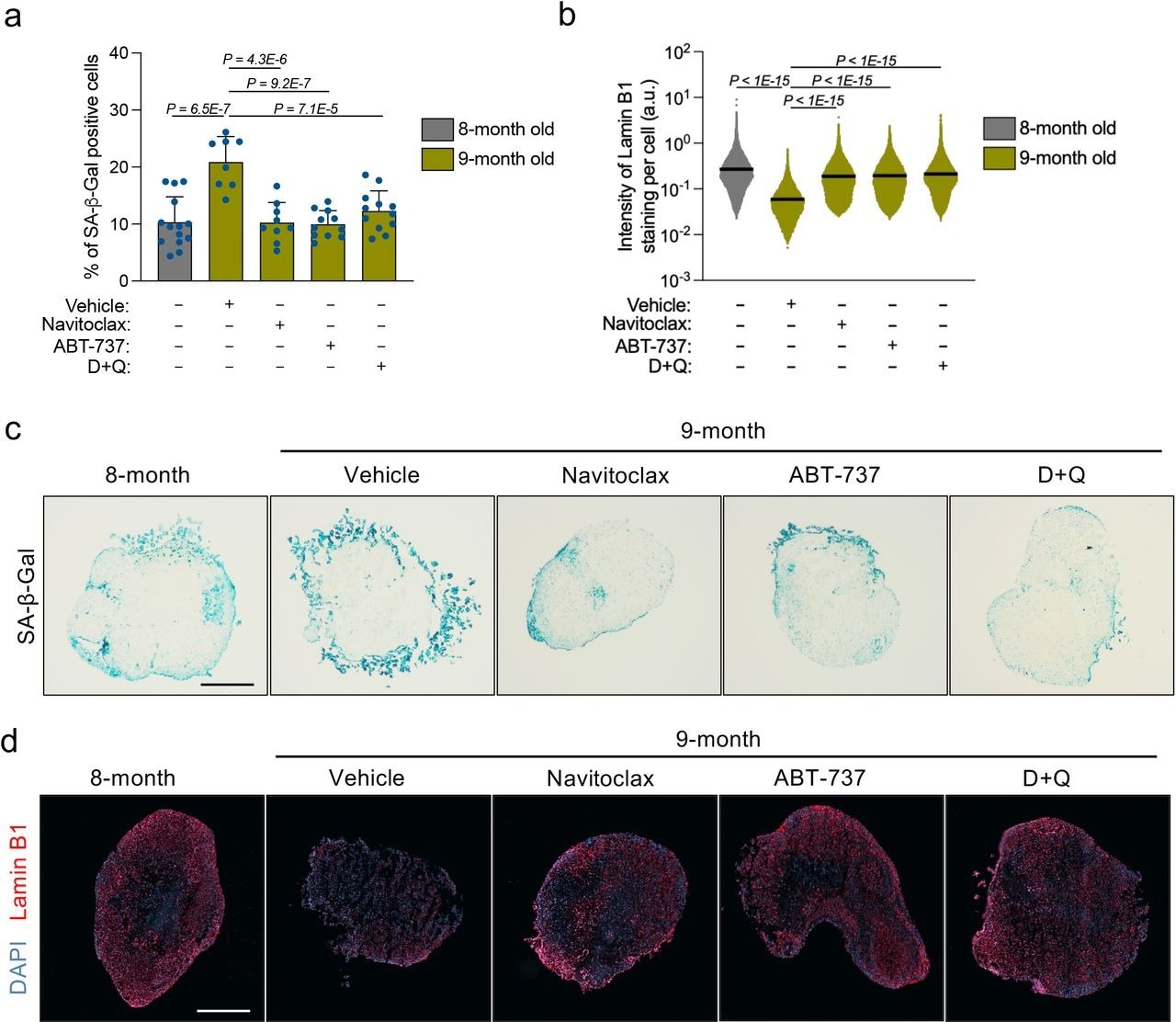 Long-term senolytic treatment prevents selective accumulation of senescent cells in physiologically aged human brain organoids. Brain organoids were generated and grown in vitro for 8 months, and subsequently exposed to two doses (each dose every two weeks) of either navitoclax (2.5 μM), ABT-737 (10 μM) or D+Q (D: 10 μM; Q: 25 μM) administration within the following month, after which the organoids were collected for in situ analysis. (a) SA-β-gal assays were performed on organoid sections. Each data point in the bar graph represents a single organoid analysed. Error bars represent s.d.; at least 8 individual organoids were analysed per condition; one-way ANOVA with Tukey’s multiple-comparison post-hoc corrections. (b) Lamin B1 staining was performed on organoid sections. Each data point in the scatter plot represents the integrated intensity of each cell within organoid sections. At least 8 individual organoids were analysed per condition; one-way ANOVA with Tukey’s multiple-comparison post-hoc corrections. (c,d) Representative images from quantifications shown in a and b, respectively. Scale bar, 0.3 mm.
Long-term senolytic treatment prevents selective accumulation of senescent cells in physiologically aged human brain organoids. Brain organoids were generated and grown in vitro for 8 months, and subsequently exposed to two doses (each dose every two weeks) of either navitoclax (2.5 μM), ABT-737 (10 μM) or D+Q (D: 10 μM; Q: 25 μM) administration within the following month, after which the organoids were collected for in situ analysis. (a) SA-β-gal assays were performed on organoid sections. Each data point in the bar graph represents a single organoid analysed. Error bars represent s.d.; at least 8 individual organoids were analysed per condition; one-way ANOVA with Tukey’s multiple-comparison post-hoc corrections. (b) Lamin B1 staining was performed on organoid sections. Each data point in the scatter plot represents the integrated intensity of each cell within organoid sections. At least 8 individual organoids were analysed per condition; one-way ANOVA with Tukey’s multiple-comparison post-hoc corrections. (c,d) Representative images from quantifications shown in a and b, respectively. Scale bar, 0.3 mm.
The brains of SARS-CoV-2 infected individuals underwent accelerated cellular senescence accumulation as compared to age-matched controls. This has been attributed to neurotropic viruses, such as the Zika virus, Japanese encephalitis virus (JEV) and SARS-CoV-2, which infects human BOs and directly induces cellular senescence.
Among the newly emerged SARS-CoV-2 variants, the Delta (B.1.617.2) variant induced the strongest cellular senescence. This observation was validated through spatial transcriptomic sequencing of p16-positive cells that detected a Delta-specific senescence-associated secretory phenotype (SASP) signature.
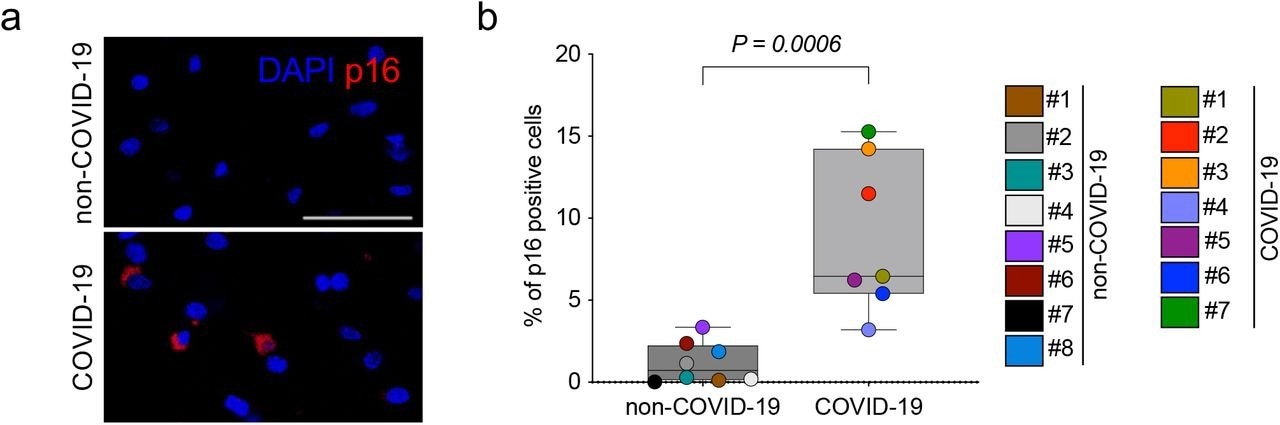 Brains of COVID-19 patients exhibit increased accumulation of p16 senescent cells. (a) Immunofluorescence images showing DAPI (blue), and p16 (red) immunoreactivity in sections of frontal cortex regions from patients with severe COVID-19 and age-matched non-COVID-related controls. Scale bar, 50 μm. (b) Box plots show the percentage of p16-positive cells. Each data point in the graph represents a single patient analysed, with a total of 2,794,379 individual brain cells across 7 COVID-19 and 8 non-COVID-19 patients. Whiskers represent min-max values; two-tailed Student’s t test.
Brains of COVID-19 patients exhibit increased accumulation of p16 senescent cells. (a) Immunofluorescence images showing DAPI (blue), and p16 (red) immunoreactivity in sections of frontal cortex regions from patients with severe COVID-19 and age-matched non-COVID-related controls. Scale bar, 50 μm. (b) Box plots show the percentage of p16-positive cells. Each data point in the graph represents a single patient analysed, with a total of 2,794,379 individual brain cells across 7 COVID-19 and 8 non-COVID-19 patients. Whiskers represent min-max values; two-tailed Student’s t test.
Five days of senolytic treatments of SARS-CoV-2-infected organoids revealed decreased viral gene expression. Additionally, this treatment inhibited the onset of senescent neurons of corticothalamic and GABAergic nature. Importantly, senolytic treatment after SARS-CoV-2 intranasal infection of K18-hACE2 mice improved COVID-19 neuropathology.
Senolytic treatment enhanced clinical scores and survival rates in SARS-CoV-2-challenged mice. An increased survival rate of dopaminergic neurons and alleviated reactive astrogliosis were also observed. Additionally, this treatment significantly decreased SASP, viral, and senescence gene expression in the brain of infected mice.
Conclusions
The current study revealed that physiologically aged human BOs accumulate senescent cells. However, senolytic treatment could effectively alleviate differential SASP expression and senescent cell burden in the treated BOs.
An intermittent senolytic treatment enhanced the clinical performance of SARS-CoV-2-infected mice, which was associated with reduced inflammation and improved survival rates of dopaminergic neurons.
Taken together, the current study indicates that senolytic therapies can alleviate long-COVID neuropathology and other disorders caused by acute neurotropic viral infections.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.