A large Finnish cohort study reveals that subtle shifts in maternal and infant gut bacteria could signal a higher risk of respiratory infections in babies, pointing to new avenues for prevention in early life.
Study: The association of maternal and infant early gut microbiota with respiratory infections in infants. Image Credit: New Africa / Shutterstock
In a recent article published in the journal Pediatric Research, researchers in Finland studied whether gut microbiota composition in mothers and young infants is linked to the risk of developing a respiratory tract infection (RTI) during the first six months of the infant’s life.
They found that infants with RTIs had differences in the relative abundance of specific bacterial taxa compared with those without, while overall alpha and beta diversity remained similar. Microbial communities in mothers also showed differences.
Background
RTIs are common in infancy. Healthy, full-term infants in high-income countries experience four to ten episodes in their first year. These infections affect infants’ well-being and have social and economic consequences. They can increase parental stress and cause missed work.
Interest is growing in how early gut microbiota might influence RTI susceptibility. Animal studies suggest gut microbes shape respiratory immunity. However, human study findings remain inconsistent.
Some research links low microbial diversity and reduced beneficial gut bacteria with higher risks of wheezing and asthma in childhood. Important bacteria include Bifidobacterium, Faecalibacterium, Ruminococcus, and Roseburia.
However, much less is known about direct associations between gut microbiota and RTIs in infancy, largely because longitudinal studies with standardized infection tracking and early-life stool sampling are limited.
Most previous studies have focused on later outcomes, such as asthma, rather than acute RTIs in early life. Furthermore, maternal microbiota, which may influence the infant gut microbiome, has rarely been examined.
About the study
Researchers hypothesized that early infant gut microbiota, along with maternal microbiota, could be associated with the occurrence of RTIs during the first six months of life. They used a nested case-control analysis that included healthy full-term non-twin Finnish infants with birth weights of at least 2.5 kg.
RTI cases were defined as infants who developed upper RTI with fever, otitis media, or lower RTI during the first six months of life. Families recorded infection symptoms and medical visits in an online diary weekly during the first four months and biweekly until seven months, allowing precise tracking of RTIs.
Fecal samples were collected from mothers around their due date and from infants at three and six weeks of age. Samples were frozen immediately at home and later processed for DNA extraction and 16S rRNA gene sequencing to characterize microbiota composition.
Of 1052 infants in the Helsinki cohort, 189 developed RTIs within six months. Microbiota data were available for 178 infants and 136 mothers in the RTI group, and for 143 infants and 125 mothers in the control group, totaling 461 infant and 261 maternal samples.
Analyses compared microbial diversity (alpha and beta) and the relative abundance of bacterial taxa between groups. Sensitivity analyses excluded infants with infections prior to stool sampling and matched cases and controls by relevant factors (season of birth, sex, and delivery mode).
Key findings
Among 178 infants who developed an RTI within the first six months and 143 controls, the median RTI duration was 11 days. Most cases were upper RTIs with fever (49%) or otitis media (47%), while only 4% were lower RTIs.
About 30% of RTI cases occurred within the first three months, most commonly otitis media. Over half of the affected infants visited a doctor, and 14% required emergency care, whereas far fewer controls had medical visits.
In maternal microbiota, overall diversity and richness did not differ between groups, but mothers of infants with RTIs had higher abundances of Citrobacter, Enterobacter, and Enterococcus, while Clostridium was lower. These bacteria were described by the authors as opportunistic pathogens, suggesting that maternal microbial instability could play a role in shaping infant risk.
For infants, overall microbial composition at 3 and 6 weeks was similar between groups. However, at three weeks, those who later developed RTIs showed higher levels of several bacterial families (such as Rikenellaceae, Prevotellaceae, and Verrucomicrobiaceae) and genera, including Alistipes, Akkermansia, Faecalibacterium, Peptoniphilus, and Serratia. The higher abundance of Faecalibacterium was notable because previous studies had often linked lower levels of this genus to respiratory problems, highlighting a potential contradiction with earlier findings.
At six weeks, Prevotellaceae remained elevated in infants who developed RTIs within three months, while reduced Anaerostipes, another butyrate producer, was observed. Anaerostipes depletion may alter lactate and butyrate metabolism, with possible downstream effects on immune function.
Sensitivity analyses confirmed these findings, showing consistent associations with higher abundances of butyrate-producing genera (Pseudobutyrivibrio, Faecalibacterium, and Roseburia), and Proteus, and lower Veillonella and Anaerostipes in infants who developed RTIs.
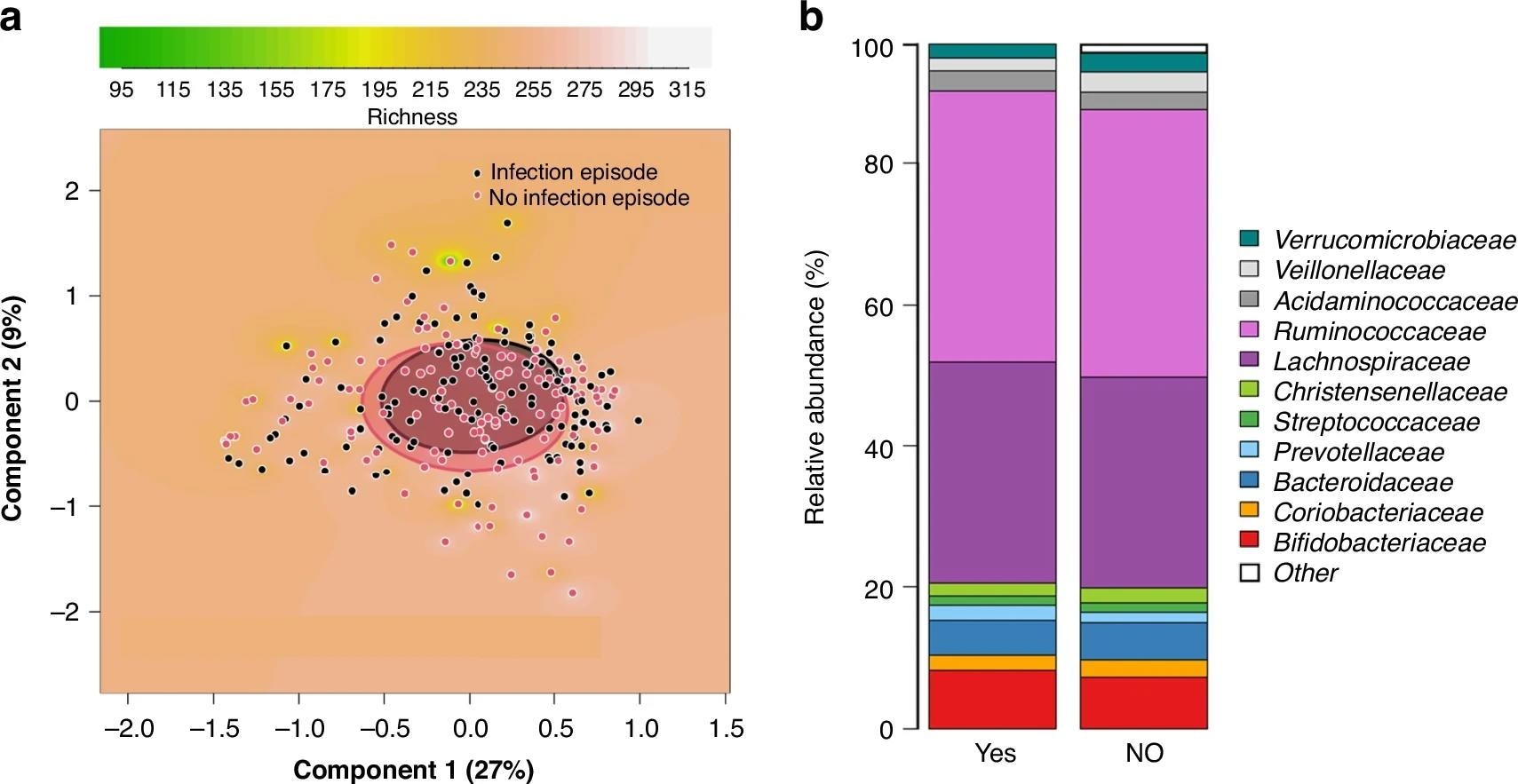
Comparison between mothers of infants who developed a respiratory tract infection episode (RTI) in the first 6 months of life and mothers of randomly selected infants with no such infection episode. PCoA plots based on Bray-Curtis dissimilarities of the samples, showing the richness of the microbiota as background (a). Clusters are shown by circles, which were drawn based on the standard deviations of the data points in each category of the samples (a). The comparisons are between mothers of infants who developed an RTI in the first 6 months of life and mothers of infants remaining healthy (p = 0.39). Clustered stacked column graphs demonstrate microbiota differences at the family level (b). The comparisons are between mothers of infants who developed an RTI in the first 6 months of life (YES) and mothers of infants remaining healthy (NO).
Conclusions
In conclusion, maternal and early infant gut microbiota composition may influence early susceptibility to RTIs, highlighting potential targets for preventive interventions in future studies. The authors emphasized these are associations, not proof of causation, and that the analysis was exploratory with a false discovery rate (FDR) threshold of 0.1. They further proposed that the early appearance of adult-type butyrate producers, such as Faecalibacterium and Roseburia, could represent a “premature gut microbiota maturation” that predisposes infants to infection.
The strengths of this analysis include the large, longitudinal birth cohort, systematic parental reporting of mild RTIs, and analysis of both infant and maternal samples. However, limitations involve the relatively homogenous, high-income population, universal breastfeeding, and restriction to healthy, full-term infants, limiting generalizability.
Note
Some microbial associations (e.g., with Faecalibacterium, Roseburia, and Pseudobutyrivibrio) were most evident in sensitivity analyses that excluded infants with early infections and matched controls by perinatal factors. These were not always present in the main unmatched analysis. Readers should interpret these findings as exploratory associations rather than consistent causal signals.
Journal reference:
- The association of maternal and infant early gut microbiota with respiratory infections in infants. Hyvönen, S., Saarikivi, A., Mälkönen, J., Solasaari, T., Korpela, K., de Vos, W.M., Salonen, A., Ruuska-Loewald, T., Kolho, K. Pediatric Research (2025). DOI: 10.1038/s41390-025-04326-0, https://www.nature.com/articles/s41390-025-04326-0