By integrating clinical assessment with cutting-edge biomarkers, researchers chart a practical pathway to faster and fairer Alzheimer’s diagnosis, ensuring patients and families gain clarity, timely care, and equal access to emerging treatments.
New landscape of the diagnosis of Alzheimer's disease. Image Credit: sfam_photo / Shutterstock
In a recent review published in The Lancet, a group of authors outlined a three-wave, clinic-based pathway that begins with clinical assessment, proceeds to staging with routine tests and MRI or CT, and confirms aetiology with first-line blood or CSF biomarkers and second-line PET when indicated to deliver earlier, accurate, and equitable diagnosis.
Background
Global prevalence estimates show Alzheimer’s disease accounts for most dementia, with ~57 million people affected in 2021 and numbers projected to triple by 2050.
People and health systems face rising costs, hard choices about new therapies, and unequal access to specialty care. Biomarkers, such as blood, cerebrospinal fluid (CSF), and positron emission tomography (PET), now elevate diagnosis from probability to biology, guiding who benefits from anti-amyloid monoclonal antibodies.
Still, implementation varies, and delays persist from first worries to confirmed diagnosis. Further research is needed to deliver fast, equitable, and affordable biomarker-based pathways for all.
Why This New Landscape Matters to People?
Timely and accurate diagnosis changes everyday life, informing decisions about driving, finances, safety at home, support at work, and when to discuss disease-modifying options. Historically, clinicians inferred Alzheimer’s disease from symptoms, bedside testing, and structural imaging; accuracy was limited because different brain diseases can look similar early on.
Molecular biomarkers like amyloid-beta (Aβ) and tau measures in CSF and blood, and amyloid/tau PET now anchor a clinical-biological diagnosis, lifting accuracy from about 60–70% to roughly 90–95% and enabling earlier confirmation in symptomatic patients. For families, “What is going on?” can finally have a biologically grounded answer that guides treatment and planning.
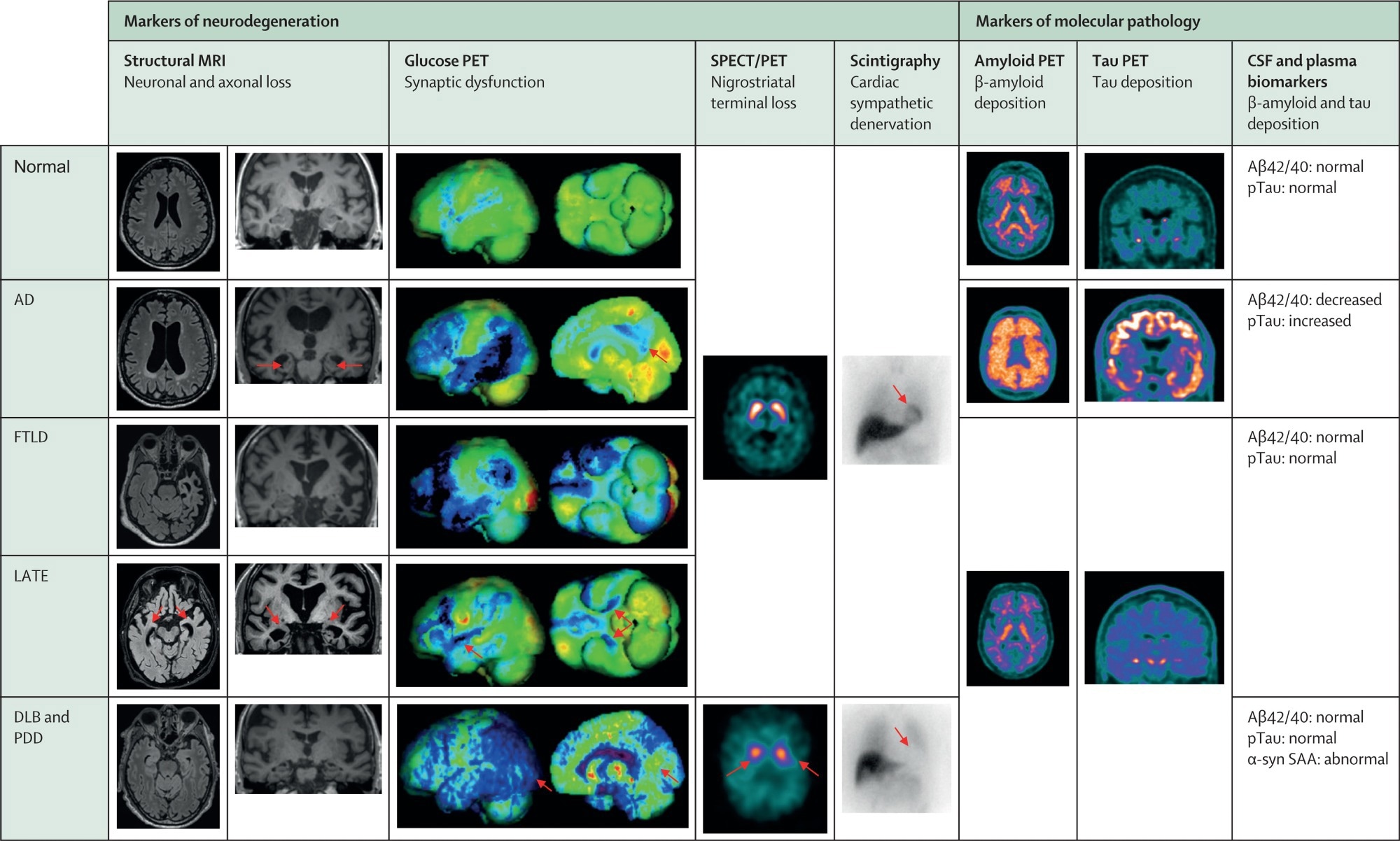
Typical biomarker profiles across pure pathology neurodegenerative cognitive disorders. Blue colour in glucose PET renderings denotes substantial hypometabolism. Orange/red/purple/white colours in nigrostriatal SPECT imaging and amyloid and tau PET denote increased tracer uptake. Images come from the archive of one of the co-authors (VG). AD=Alzheimer's disease with typical amnestic phenotype. CSF=cerebrospinal fluid. DLB=dementia with Lewy bodies. FTLD=frontotemporal lobar degeneration with behavioural phenotype. LATE=limbic-predominant age-related TDP-43 encephalopathy. PDD=Parkinson's disease dementia. SAA=seed amplification assay. SPECT=single-photon emission computed tomography.
Incidence And Impact for Families and Policy
Incidence climbs steeply with age, and dementia prevalence is expected to triple by mid-century. Disparities persist by sex, race, and ethnicity. In the United States, African Americans experience around 27 dementia cases per 1,000 person-years compared with about 19 for White Americans.
Much of the risk gap tracks cardiovascular factors and social determinants of health (education, neighborhood, pollution, access to care). Encouragingly, age-specific incidence appears to be declining in some high-income countries, likely due to higher education and better cardiovascular risk control, but these gains are fragile and may reverse with rising obesity, sedentary behavior, and type 2 diabetes mellitus.
For individuals, this translates into the importance of maintaining lifelong brain health habits alongside access to accurate diagnosis.
The Real-World Patient Journey: Three “Waves,” One Goal
Memory clinics increasingly follow a three-wave workflow. Wave 1 spans history-taking, brief cognitive screening, and neurologic exam, sorting people into cognitively unimpaired versus impaired and ruling out obvious secondary causes.
Wave 2 completes staging with a cognitive battery, routine laboratory tests, and Magnetic Resonance Imaging (MRI) or Computed Tomography (CT) scans, including assessment for delirium, and excludes other non-neurodegenerative causes. Clinicians make a syndromic diagnosis (e.g., amnestic, language, visuospatial) and an aetiologic hypothesis.
Wave 3 tests that hypothesis using first-line and second-line biomarkers to reach a molecular diagnosis (for Alzheimer’s disease) or a topographic diagnosis (for other proteinopathies). This staged approach standardizes care and reduces the likelihood of misdiagnosis.
Short cognitive tools, such as the Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), and General Practitioner Assessment of Cognition, nearly double recognition compared with unaided clinical impressions, enabling earlier referrals. These tools are complemented by culturally fair options (e.g., Rowland Universal Dementia Assessment Scale) as systems move toward broader, earlier detection.
PET and CSF: What They Tell and Do not Tell
Amyloid PET tracers ([¹⁸F]-florbetapir, florbetaben, flutemetamol) visualize Aβ plaque burden on a standard centiloid scale, while tau PET shows neocortical tau that correlates closely with clinical stage and cognitive profile.
In CSF, decreased Aβ42:Aβ40 and increased phosphorylated-tau (p-tau181) reflect amyloid/tau dysregulation. United States Food and Drug Administration (FDA)-cleared CSF tests use ratios (Aβ42:Aβ40, p-tau181:Aβ42, total-tau: Aβ42) rather than Aβ42 alone.
PET images insoluble aggregates and maps topography; CSF reflects soluble pathway changes, often earlier. Amyloid PET typically becomes clearly positive at moderate plaque burden, whereas CSF or blood markers can shift earlier in the disease course. Together, they clarify who truly has Alzheimer’s disease pathology when symptoms are still mild.
Blood Biomarkers: Opening The Door in Routine Care
Plasma p-tau217 assays now demonstrate high positive and negative predictive values for underlying amyloid/tau pathology, reducing the need for CSF and PET by approximately 80–90% when used early in the diagnostic pathway.
Crucially, the first FDA-cleared blood-based in vitro diagnostic device (Lumipulse p-tau217:Aβ42 ratio) is now available for symptomatic patients. At present, these blood tests are clinically available in the USA and a few other countries, but not yet globally.
As these tests become more widespread, primary care triage can improve, specialty bottlenecks may ease, and more patients can be assessed earlier and more fairly. Clinicians should still consider comorbidities (e.g., chronic kidney disease can raise p-tau217) and, when needed, confirm borderline or intermediate results with a second modality.
Neurodegeneration Markers: Linking Biology to Daily Function
Neurodegeneration connects pathology to symptoms. MRI patterns (e.g., medial temporal atrophy) and [¹⁸F]-fluorodeoxyglucose PET (FDG-PET) hypometabolism help document topography and severity; FDG-PET is often more sensitive than atrophy in the early stages.
Plasma or CSF neurofilament light further flags axonal injury across neurodegenerative diseases and helps distinguish frontotemporal degeneration from Alzheimer’s disease when combined with p-tau217. For patients, these tests explain “why memory fails” and help anticipate needs at home while clinicians target the right disease mechanism.
Treatments Make Confirmation Essential and Raise Equity Questions
Anti-amyloid monoclonal antibodies like lecanemab and donanemab are now approved in multiple regions (European Union, United States of America, United Kingdom, China, Japan, South Korea, Hong Kong, United Arab Emirates, Israel). Therapy eligibility requires Aβ confirmation, moving biomarker testing from “useful” to indispensable.
Systems must therefore scale testing, streamline referrals, and protect access for rural and underserved communities so that treatment opportunities do not widen disparities. The authors also caution that frailty in older adults and the underrepresentation of some racial and ethnic groups in validation cohorts should be considered when interpreting results and determining treatment eligibility.
Conclusions
This Series paper outlines a practical, three-wave pathway that integrates clinical expertise with biomarkers to facilitate earlier and more accurate diagnosis of Alzheimer’s disease. Blood p-tau217 testing can efficiently triage, while CSF ratios and amyloid/tau PET confirm biology and map topography. Neurodegeneration measures link pathology to day-to-day function.
Because anti-amyloid therapy requires Aβ confirmation, biomarker access is now central to equitable care. The authors emphasize the importance of judicious test interpretation, attention to comorbidities and diversity, and clear communication with patients and their families. Implemented effectively, this approach reduces misdiagnosis, informs treatment decisions, and enables people to plan their futures with confidence.
Journal reference:
- Frisoni, G. B., Hansson, O., Nichols, E., Garibotto, V., Schindler, S. E., van der Flier, W. M., Jessen, F., Villain, N., Arenaza-Urquijo, E. M., Crivelli, L., Fortea, J., Grinberg, L. T., Ismail, Z., Minoshima, S., Ossenkoppele, R., Zetterberg, H., Petersen, R. C., & Dubois, B. (2025). New landscape of the diagnosis of Alzheimer's disease. The Lancet. DOI: 10.1016/S0140-6736(25)01294-2 https://www.thelancet.com/journals/lancet/article/PIIS0140-6736%2825%2901294-2/fulltext