From delaying the onset of type 1 diabetes with immunotherapy to restoring insulin production with stem cells and easing life through smart delivery systems, a new era of type 1 diabetes treatment is emerging, but only if access and equity keep pace.
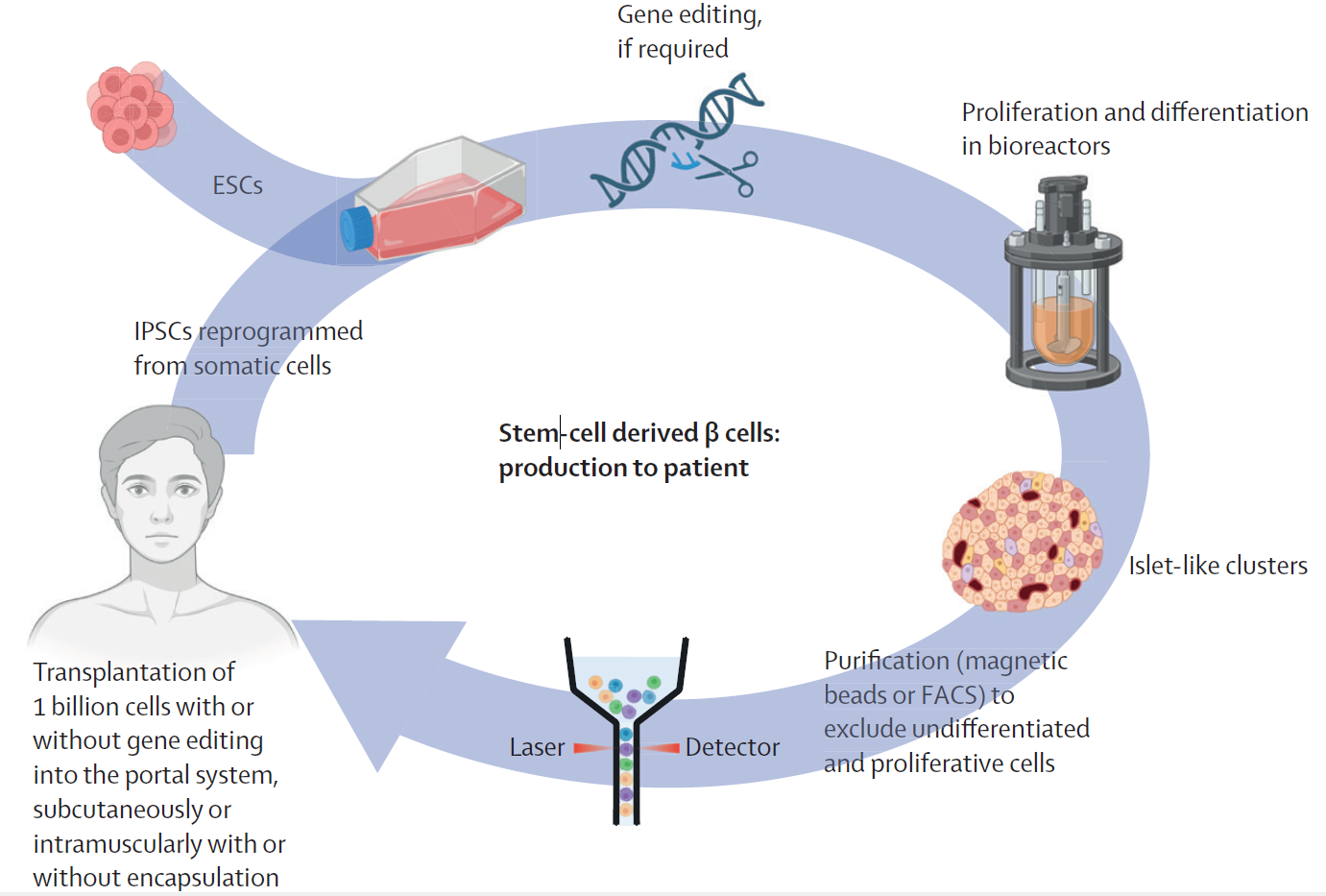
Production and transplantation of β cells derived from embryonic or induced pluripotent stem cells ESCs=embryonic stem cells. FACS=fluorescence-activated cell sorter. IPSCs=induced pluripotent stem cells.
In a recent review published in The Lancet, a group of authors synthesized contemporary evidence on disease-modifying immunotherapies, β-cell replacement strategies, and automated insulin delivery to clarify benefits, risks, access barriers, and priorities for future clinical translation in type 1 diabetes.
Background
One diagnosis, lifelong decisions: families plan meals, school, travel, and sports around glucose checks and injections. Despite modern care, people diagnosed with type 1 diabetes still face reduced life expectancy and high complication risk. Screening for islet autoantibodies now detects presymptomatic disease, while the United States Food and Drug Administration (FDA) approval of teplizumab, an anti-Cluster of Differentiation 3 (CD3) monoclonal antibody, demonstrates that immunotherapy can delay the clinical onset. Follow-up data indicate that the median delay extends to approximately 32 months. At the same time, stem-cell-derived β-cells and automated insulin delivery promise better control and quality of life. Yet costs, access, durability, and safety remain uneven. Further research is needed to deliver scalable, equitable, and lasting disease modification.
Screening and Early Diagnosis
Type 1 diabetes unfolds in stages that can be identified before symptoms appear. Stage 1 features normoglycemia with two or more islet autoantibodies; Stage 2 adds dysglycemia; Stage 3 is characterized by clinical hyperglycemia requiring insulin. Early identification through certified islet-autoantibody testing reduces diabetic ketoacidosis at diagnosis and, crucially, opens the door to disease-modifying therapy. Risk stratification integrates antibody titers, glycemic markers, body mass index (BMI), and connecting peptide (C-peptide). As public health programs expand screening beyond relatives to the general pediatric population, standardized master protocols and guidance from professional societies are essential to ensure accuracy, follow-up, and ethical implementation.
Disease-Modifying Immunotherapies
Teplizumab (anti-CD3) is the first therapy to delay clinical onset in Stage 2, with a median delay of about 24 months and up to 32 months in extended follow-up after a single 14-day intravenous course. Mechanistically, it induces transient lymphopenia and shifts T-cell phenotypes toward exhaustion and anergy, thereby reducing autoimmune attacks on pancreatic β-cells. Safety signals include rash, headache, gastrointestinal symptoms, transient elevations in liver enzymes, and mild cytokine-release syndromes, most of which are manageable.
Evidence from recent-onset Stage 3 trials (for example, the PROTECT trial) shows preservation of meal-stimulated C-peptide, suggesting that benefits are greatest when treatment starts early. Other candidates target T-cell costimulation (abatacept, a cytotoxic T-lymphocyte-associated protein-4 immunoglobulin [CTLA-4-Ig]), cytokine pathways (ustekinumab for interleukin-12/interleukin-23), B-cells (rituximab), and broad immune signaling (baricitinib, a Janus kinase [JAK] inhibitor), as well as low-dose anti-thymocyte globulin (ATG) and tumor necrosis factor (TNF) inhibitors (golimumab). These agents variably preserve β-cell function, reduce insulin needs, and improve glycated hemoglobin (HbA1c), though durability and regulatory approvals are pending. Strategic combinations, including immune and non-immune modulators such as verapamil, are being investigated to enhance the magnitude and persistence of the effect, although pivotal trials are still ongoing. Antigen-based therapies such as oral insulin or GAD-alum have shown limited or subgroup-specific efficacy.
β-Cell Replacement and Stem-Cell Strategies
Replacement therapy aims to restore endogenous insulin secretion rather than merely supplement it. Pancreas transplantation, often simultaneous with kidney transplantation, achieves high insulin independence but requires systemic immunosuppression and has perioperative risks. Allogeneic islet transplantation helps patients with severe hypoglycemia and hypoglycemia unawareness, yet long-term function can wane due to alloimmunity, recurrent autoimmunity, and drug toxicity, and donor scarcity limits access.
A breakthrough comes from embryonic stem-cell-derived β-cells such as Vertex-880, infused into the portal vein under full immunosuppression, which have shown high insulin-independence rates in early cohorts at about one year, with durability still under investigation. Autologous, induced pluripotent stem cell-derived β-cells have achieved insulin independence in early individual cases, with longer-term outcomes pending, which could potentially reduce alloimmune barriers. To avoid chronic immunosuppression, two paths are advancing: encapsulation devices that physically shield grafts and genome-edited “hypoimmune” cells (e.g., β-2-microglobulin or class II transactivator edits, CD47 overexpression) to blunt immune recognition. Remaining challenges include tumorigenicity from undifferentiated cells, industrial-scale manufacturing (~1 billion cells per person), bioreactor optimization, cost containment, and robust long-term safety monitoring.
Innovations in Insulin and Delivery
New-generation insulins seek physiology-like action. Ultrarapid analogs (e.g., faster-acting insulin aspart, ultrarapid-acting insulin lispro) accelerate post-meal control without sacrificing overall safety. Inhaled human insulin (Afrezza) offers the fastest onset of action through lung absorption and is currently being studied in children. Once-weekly basal insulins (insulin icodec, insulin efsitora alfa) match the efficacy of daily degludec, although insulin icodec carries a higher rate of clinically significant hypoglycemia, requiring vigilance. Glucose-responsive insulins, or “smart” formulations that release insulin in hyperglycemia, remain investigational.
Automated insulin delivery (AID) systems couple continuous glucose monitors (CGMs) with pump algorithms to adjust dosing in real-time, improving time-in-range by ~10-15 percentage points across all age groups and reducing HbA1c without increasing hypoglycemia. One AID system is approved for use during pregnancy, where it improves the time spent in the tighter 3.5–7.8 mmol/L range without increasing the incidence of hypoglycemia. Emerging features include meal-detection algorithms, multi-hormone systems (such as insulin with glucagon or amylin analogs), and adjunctive agents, including GLP-1 receptor agonists and SGLT2 inhibitors. However, only pramlintide is FDA-approved for T1D, and SGLT2 inhibitors increase the risk of diabetic ketoacidosis.
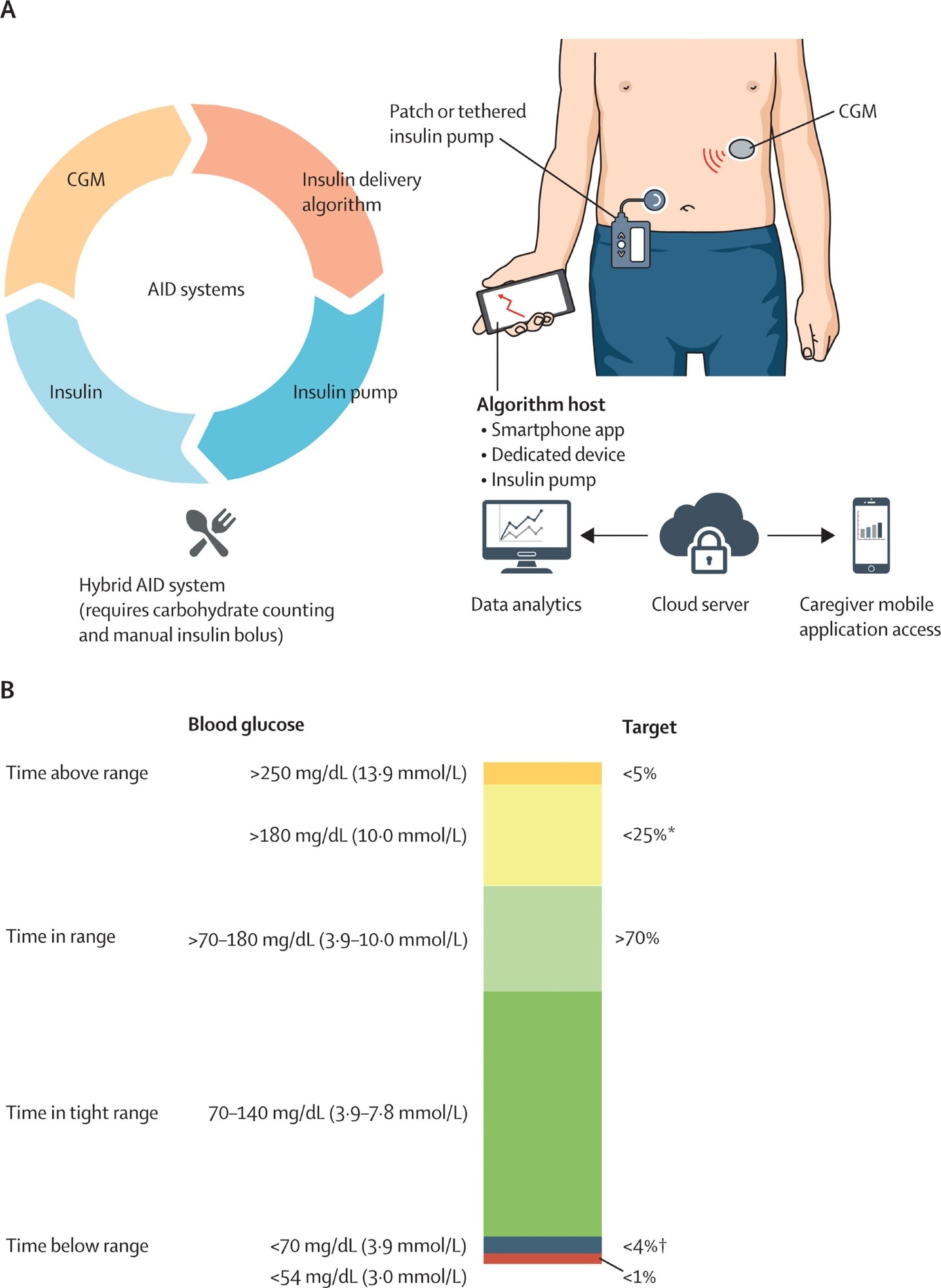
AID systems and CGM-based glycaemia targets (A) AID system with its connected components. Insulin dose is calculated on the basis of CGM values using an insulin delivery algorithm, and insulin is infused by the insulin infusion pump (tethered or patch pump). Dual-hormone systems (investigational and not depicted in the figure) infuse other hormones such as glucagon in addition to insulin. Hybrid AID requires meal-time carbohydrate counting and meal bolus input. CGM and insulin delivery data are uploaded to the cloud server for data analytics and can be accessed by parents, caregivers, and clinicians of people with diabetes. (B) Percentage of readings and time per day within target glucose range, time below target glucose range, time above target glucose range, and time in tight range. AID=automated insulin delivery. CGM=continuous glucose monitor. *Includes percentage of values <250 mg/dL (13·9 mmol/L). †Includes percentage of values <54 mg/dL (3·0 mmol/L).
Access, Equity, and Regulation
Innovations mean little without access. Many families, especially in low-income or migrant communities, lack insurance coverage for autoantibody screening, teplizumab, CGM, pumps, or training. Policies that reimburse diagnostics and therapies, provide multilingual education, and foster community partnerships can help narrow disparities. Regulators currently prioritize hard outcomes (clinical onset delay, insulin dose reduction, HbA1c), yet firm evidence links higher C-peptide to fewer hypoglycemic events and long-term complications. Recognizing C-peptide as a validated surrogate endpoint could accelerate approvals and investment while maintaining safety standards.
Future Directions
Personalized immunotherapy, guided by human leukocyte antigen (HLA) haplotypes, antibody profiles, and immune phenotypes, may identify “likely responders” and refine timing (e.g., Stage 2 vs early Stage 3). Combination regimens, sequential biologics, and protective non-immune agents could extend remission. On the curative front, safe, scalable, gene-edited β-cells that resist both alloimmunity and autoimmunity, coupled with affordable manufacturing, remain the goal. In parallel, fully automated AID that eliminates meal boluses could lighten the daily burden for most people living with diabetes.
Conclusions
The therapeutic horizon for type 1 diabetes is no longer confined to better injections. Disease-modifying immunotherapy, such as teplizumab, demonstrates that delaying clinical onset is feasible; broader pipelines targeting T- and B-cell pathways, cytokines, and signaling may further preserve β-cells. Stem-cell-derived β-cells can restore endogenous insulin production, with engineering advances aiming to eliminate the need for lifelong immunosuppression. Meanwhile, automated insulin delivery, ultrarapid and once-weekly insulins, and adjuncts modernize daily care. Translating these gains into population-level benefit will require equitable screening, coverage, and regulatory flexibility, including wider acceptance of C-peptide as a surrogate endpoint to expedite safe and durable access.